Cephalosporin-3'-Diazeniumdiolate NO Donor Prodrug PYRRO-C3D Enhances Azithromycin Susceptibility of Nontypeable Haemophilus influenzae Biofilms
- PMID: 27919896
- PMCID: PMC5278716
- DOI: 10.1128/AAC.02086-16
Cephalosporin-3'-Diazeniumdiolate NO Donor Prodrug PYRRO-C3D Enhances Azithromycin Susceptibility of Nontypeable Haemophilus influenzae Biofilms
Abstract
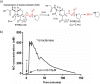
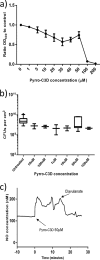
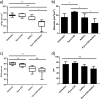
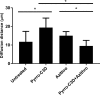
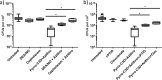
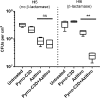
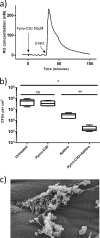
PYRRO-C3D is a cephalosporin-3-diazeniumdiolate nitric oxide (NO) donor prodrug designed to selectively deliver NO to bacterial infection sites. The objective of this study was to assess the activity of PYRRO-C3D against nontypeable Haemophilus influenzae (NTHi) biofilms and examine the role of NO in reducing biofilm-associated antibiotic tolerance. The activity of PYRRO-C3D on in vitro NTHi biofilms was assessed through CFU enumeration and confocal microscopy. NO release measurements were performed using an ISO-NO probe. NTHi biofilms grown on primary ciliated respiratory epithelia at an air-liquid interface were used to investigate the effects of PYRRO-C3D in the presence of host tissue. Label-free liquid chromatography-mass spectrometry (LC/MS) proteomic analyses were performed to identify differentially expressed proteins following NO treatment. PYRRO-C3D specifically released NO in the presence of NTHi, while no evidence of spontaneous NO release was observed when the compound was exposed to primary epithelial cells. NTHi lacking β-lactamase activity failed to trigger NO release. Treatment significantly increased the susceptibility of in vitro NTHi biofilms to azithromycin, causing a log fold reduction (10-fold reduction or 1-log-unit reduction) in viability (P < 0.05) relative to azithromycin alone. The response was more pronounced for biofilms grown on primary respiratory epithelia, where a 2-log-unit reduction was observed (P < 0.01). Label-free proteomics showed that NO increased expression of 16 proteins involved in metabolic and transcriptional/translational functions. NO release from PYRRO-C3D enhances the efficacy of azithromycin against NTHi biofilms, putatively via modulation of NTHi metabolic activity. Adjunctive therapy with NO mediated through PYRRO-C3D represents a promising approach for reducing biofilm-associated antibiotic tolerance.
Keywords: Haemophilus influenzae; antibiotic resistance; biofilms; nitric oxide; proteomics.
Copyright © 2017 American Society for Microbiology.
Figures
References
-
- Ramsey KA, Ranganathan S, Park J, Skoric B, Adams AM, Simpson SJ, Robins-Browne RM, Franklin PJ, de Klerk NH, Sly PD, Stick SM, Hall GL, Arest CF. 2014. Early respiratory infection is associated with reduced spirometry in children with cystic fibrosis. Am J Respir Crit Care Med 190:1111–1116. doi:10.1164/rccm.201407-1277OC. - DOI - PubMed
-
- Rogers GB, Carroll MP, Zain NMM, Bruce KD, Lock K, Walker W, Jones G, Daniels TWV, Lucas JS. 2013. Complexity, temporal stability, and clinical correlates of airway bacterial community composition in primary ciliary dyskinesia. J Clin Microbiol 51:4029–4035. doi:10.1128/JCM.02164-13. - DOI - PMC - PubMed
-
- Hall-Stoodley L, Hu FZ, Gieseke A, Nistico L, Nguyen D, Hayes J, Forbes M, Greenberg DP, Dice B, Burrows A, Wackym PA, Stoodley P, Post JC, Ehrlich GD, Kerschner JE.. 2006. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA 296:202–211. doi:10.1001/jama.296.2.202. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

