Interleukin-37 and Dendritic Cells Treated With Interleukin-37 Plus Troponin I Ameliorate Cardiac Remodeling After Myocardial Infarction
- PMID: 27919929
- PMCID: PMC5210436
- DOI: 10.1161/JAHA.116.004406
Interleukin-37 and Dendritic Cells Treated With Interleukin-37 Plus Troponin I Ameliorate Cardiac Remodeling After Myocardial Infarction
Abstract
Background: Excessive immune-mediated inflammatory reactions play a deleterious role in postinfarction ventricular remodeling. Interleukin-37 (IL-37) emerges as an inhibitor of both innate and adaptive immunity. However, the exact role of IL-37 and IL-37 plus troponin I (TnI)-treated dendritic cells (DCs) in ventricular remodeling after myocardial infarction (MI) remains elusive.
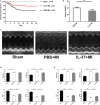
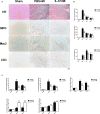
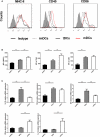
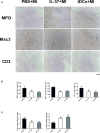
Methods and results: MI was induced by permanent ligation of the left anterior descending artery. Our results showed that treatment with recombinant human IL-37 significantly ameliorated ventricular remodeling after MI, as demonstrated by decreased infarct size, better cardiac function, lower mortality, restricted inflammatory responses, decreased myocardial fibrosis, and inhibited cardiomyocyte apoptosis. In vitro, we examined the phenotype of IL-37 plus TnI-conditioned DCs of male C57BL/6 mice and their capacity to influence the number of regulatory T cells. Our results revealed that IL-37 plus TnI-conditioned DCs obtained the characteristics of tolerogenic DCs (tDCs) and expanded the number of regulatory T cells when co-cultured with splenic CD4+ T cells. Interestingly, we also found that adoptive transfer of these antigen-loaded tDCs markedly increased the number of regulatory T cells in the spleen, attenuated the infiltration of inflammatory cells in the infarct hearts, decreased myocardial fibrosis, and improved cardiac function.
Conclusions: Our results reveal a beneficial role of IL-37 or tDCs treated with IL-37 plus TnI in post-MI remodeling that is possibly mediated by reestablishing a tolerogenic immune response, indicating that IL-37 or adoptive transfer of IL-37 plus TnI-treated tDCs may be a novel therapeutic strategy for ventricular remodeling after MI.
Keywords: Treg cells; interleukin‐37; myocardial infarction; remodeling; tolerogenic dendritic cells.
© 2016 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell.
Figures








References
-
- Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res. 2004;94:1543–1553. - PubMed
-
- Timmers L, Sluijter JP, van Keulen JK, Hoefer IE, Nederhoff MG, Goumans MJ, Doevendans PA, van Echteld CJ, Joles JA, Quax PH, Piek JJ, Pasterkamp G, de Kleijn DP. Toll‐like receptor 4 mediates maladaptive left ventricular remodeling and impairs cardiac function after myocardial infarction. Circ Res. 2008;102:257–264. - PubMed
-
- Zhou SF, Yuan J, Liao MY, Xia N, Tang TT, Li JJ, Jiao J, Dong WY, Nie SF, Zhu ZF, Zhang WC, Lv BJ, Xiao H, Wang Q, Tu X, Liao YH, Shi GP, Cheng X. IL‐17A promotes ventricular remodeling after myocardial infarction. J Mol Med (Berl). 2014;92:1105–1116. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

