TGF-β Family Signaling in the Control of Cell Proliferation and Survival
- PMID: 27920038
- PMCID: PMC5378054
- DOI: 10.1101/cshperspect.a022145
TGF-β Family Signaling in the Control of Cell Proliferation and Survival
Abstract
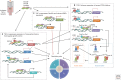
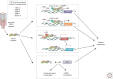
The transforming growth factor β (TGF-β) family controls many fundamental aspects of cellular behavior. With advances in the molecular details of the TGF-β signaling cascade and its cross talk with other signaling pathways, we now have a more coherent understanding of the cytostatic program induced by TGF-β. However, the molecular mechanisms are still largely elusive for other cellular processes that are regulated by TGF-β and determine a cell's proliferation and survival, apoptosis, dormancy, autophagy, and senescence. The difficulty in defining TGF-β's roles partly stems from the context-dependent nature of TGF-β signaling. Here, we review our current understanding and recent progress on the biological effects of TGF-β at the cellular level, with the hope of providing a framework for understanding how cells respond to TGF-β signals in specific contexts, and why disruption of such mechanisms may result in different human diseases including cancer.
Copyright © 2017 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
