Regulation of TGF-β Family Signaling by Inhibitory Smads
- PMID: 27920040
- PMCID: PMC5334261
- DOI: 10.1101/cshperspect.a022095
Regulation of TGF-β Family Signaling by Inhibitory Smads
Abstract
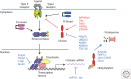
Inhibitory Smads (I-Smads) have conserved carboxy-terminal MH2 domains but highly divergent amino-terminal regions when compared with receptor-regulated Smads (R-Smads) and common-partner Smads (co-Smads). Smad6 preferentially inhibits Smad signaling initiated by the bone morphogenetic protein (BMP) type I receptors ALK-3 and ALK-6, whereas Smad7 inhibits both transforming growth factor β (TGF-β)- and BMP-induced Smad signaling. I-Smads also regulate some non-Smad signaling pathways. Here, we discuss the vertebrate I-Smads, their roles as inhibitors of Smad activation and regulators of receptor stability, as scaffolds for non-Smad signaling, and their possible roles in the nucleus. We also discuss the posttranslational modification of I-Smads, including phosphorylation, ubiquitylation, acetylation, and methylation.
Copyright © 2017 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures


References
-
- Afrakhte M, Morén A, Jossan S, Itoh S, Sampath K, Westermark B, Heldin CH, Heldin NE, ten Dijke P. 1998. Induction of inhibitory Smad6 and Smad7 mRNA by TGF-β family members. Biochem Biophys Res Commun 249: 505–511. - PubMed
-
- Arnold NB, Ketterer K, Kleeff J, Friess H, Buchler MW, Korc M. 2004. Thioredoxin is downstream of Smad7 in a pathway that promotes growth and suppresses cisplatin-induced apoptosis in pancreatic cancer. Cancer Res 64: 3599–3606. - PubMed
-
- Azuma H, Ehata S, Miyazaki H, Watabe T, Maruyama O, Imamura T, Sakamoto T, Kiyama S, Kiyama Y, Ubai T, et al. 2005. Smad7 inhibits metastasis of mouse breast cancer by direct action on cancer cells. J Natl Cancer Inst 97: 1734–1746. - PubMed
-
- Bachmaier K, Krawczyk C, Kozieradzki I, Kong YY, Sasaki T, Oliveira-dos-Santos A, Mariathasan S, Bouchard D, Wakeham A, Itie A, et al. 2000. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature 403: 211–216. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
