New concepts for an old problem: the diagnosis of endometrial hyperplasia
- PMID: 27920066
- PMCID: PMC5850217
- DOI: 10.1093/humupd/dmw042
New concepts for an old problem: the diagnosis of endometrial hyperplasia
Abstract
Background: Endometrial hyperplasia (EH) is a uterine pathology representing a spectrum of morphological endometrial alterations. It is predominantly characterized by an increase in the endometrial gland-to-stroma ratio when compared to normal proliferative endometrium. The clinical significance of EH lies in the associated risk of progression to endometrioid endometrial cancer (EC) and 'atypical' forms of EH are regarded as premalignant lesions. Traditional histopathological classification systems for EH exhibit wide and varying degrees of diagnostic reproducibility and, as a consequence, standardized patient management can be challenging.
Objective and rationale: EC is the most common gynaecological malignancy in developed countries. The incidence of EC is rising, with alarming increases described in the 40-44-year-old age group. This review appraises the current EH classification systems used to stratify women at risk of malignant progression to EC. In addition, we summarize the evidence base regarding the use of immunohistochemical biomarkers for EH and discuss an emerging role for genomic analysis.
Search methods: PubMed, Medline and the Cochrane Database were searched for original peer-reviewed primary and review articles, from January 2000 to January 2016. The following search terms were used: 'endometrial hyperplasia', 'endometrial intraepithelial neoplasia', 'atypical hyperplasia', 'complex atypical hyperplasia', 'biomarker', 'immunohistochemistry', 'progression', 'genomic', 'classification' and 'stratification'.
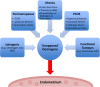
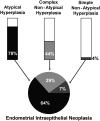
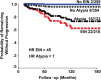
Outcomes: Recent changes to EH classification reflect our current understanding of the genesis of endometrioid ECs. The concept of endometrial intraepithelial neoplasia (EIN) as a mutationally activated, monoclonal pre-malignancy represents a fundamental shift from the previously held notion that unopposed oestrogenic stimulation causes ever-increasing hyperplastic proliferation, with accumulating cytological atypia that imperceptibly leads to the development of endometrioid EC. Our review highlights several key biomarker candidates that have been described as both diagnostic tools for EH and markers of progression to EC. We propose that, moving forwards, a 'panel' approach of combinations of the immunohistochemical biomarkers described in this review may be more informative since no single candidate can currently fill the entire role.
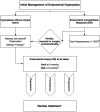
Wider implications: EC has historically been considered a predominantly postmenopausal disease. Owing in part to the current unprecedented rates of obesity, we are starting to see signs of a shift towards a rising incidence of EC amongst pre- and peri-menopausal woman. This creates unique challenges both diagnostically and therapeutically. Furthering our understanding of the premalignant stages of EC development will allow us to pursue earlier diagnosis and facilitate appropriate stratification of women at risk of developing EC, permitting timely and appropriate therapeutic interventions.
Keywords: biomarkers; endometrial hyperplasia; endometrial intraepithelial neoplasia; endometrioid endometrial cancer; genomic classification; immunohistochemistry; patient stratification; personalized medicine; progression.
© The Author 2016. Published by Oxford University Press on behalf of the European Society of Human Reproduction and Embryology.
Figures





References
-
- Abu-Rustum NR, Zhou Q, Gomez JD, Alektiar KM, Hensley ML, Soslow RA, Levine DA, Chi DS, Barakat RR, Iasonos A. A nomogram for predicting overall survival of women with endometrial cancer following primary therapy: toward improving individualized cancer care. Gynecol Oncol 2010;116:399–403. - PMC - PubMed
-
- Ahmed ARH, Muhammad EMS. E-cadherin and CD10 expression in atypical hyperplastic and malignant endometrial lesions. J Egypt Natl Canc Inst 2014;26:211–217. - PubMed
-
- Allison KH, Tenpenny E, Reed SD, Swisher EM, Garica RL. Immunohistochemical markers in endometrial hyperplasia: is there a panel with promise. Appl Immunohistochem Mol Morphol 2008;16:329–343. - PubMed
-
- Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet 2005;366:491–505. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

