Interferon-γ Limits Diabetogenic CD8+ T-Cell Effector Responses in Type 1 Diabetes
- PMID: 27920091
- PMCID: PMC5319715
- DOI: 10.2337/db16-0846
Interferon-γ Limits Diabetogenic CD8+ T-Cell Effector Responses in Type 1 Diabetes
Abstract
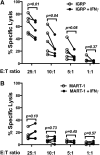
Type 1 diabetes development in the NOD mouse model is widely reported to be dependent on high-level production by autoreactive CD4+ and CD8+ T cells of interferon-γ (IFN-γ), generally considered a proinflammatory cytokine. However, IFN-γ can also participate in tolerance-induction pathways, indicating it is not solely proinflammatory. This study addresses how IFN-γ can suppress activation of diabetogenic CD8+ T cells. CD8+ T cells transgenically expressing the diabetogenic AI4 T-cell receptor adoptively transferred disease to otherwise unmanipulated NOD.IFN-γnull , but not standard NOD, mice. AI4 T cells only underwent vigorous intrasplenic proliferation in NOD.IFN-γnull recipients. Disease-protective IFN-γ could be derived from any lymphocyte source and suppressed diabetogenic CD8+ T-cell responses both directly and through an intermediary nonlymphoid cell population. Suppression was not dependent on regulatory T cells, but was associated with increased inhibitory STAT1 to STAT4 expression levels in pathogenic AI4 T cells. Importantly, IFN-γ exposure during activation reduced the cytotoxicity of human-origin type 1 diabetes-relevant autoreactive CD8+ T cells. Collectively, these results indicate that rather than marking the most proinflammatory lymphocytes in diabetes development, IFN-γ production could represent an attempted limitation of pathogenic CD8+ T-cell activation. Thus, great care should be taken when designing possible diabetic intervention approaches modulating IFN-γ production.
© 2017 by the American Diabetes Association.
Figures



Similar articles
-
The Presence and Preferential Activation of Regulatory T Cells Diminish Adoptive Transfer of Autoimmune Diabetes by Polyclonal Nonobese Diabetic (NOD) T Cell Effectors into NSG versus NOD-scid Mice.J Immunol. 2015 Oct 1;195(7):3011-9. doi: 10.4049/jimmunol.1402446. Epub 2015 Aug 17. J Immunol. 2015. PMID: 26283479 Free PMC article.
-
IFN-γ receptor deficiency prevents diabetes induction by diabetogenic CD4+, but not CD8+, T cells.Eur J Immunol. 2012 Aug;42(8):2010-8. doi: 10.1002/eji.201142374. Eur J Immunol. 2012. PMID: 22865049 Free PMC article.
-
All-trans retinoic acid inhibits type 1 diabetes by T regulatory (Treg)-dependent suppression of interferon-gamma-producing T-cells without affecting Th17 cells.Diabetes. 2009 Jan;58(1):146-55. doi: 10.2337/db08-1154. Epub 2008 Nov 4. Diabetes. 2009. PMID: 18984738 Free PMC article.
-
Inflammation versus regulation: how interferon-gamma contributes to type 1 diabetes pathogenesis.Front Cell Dev Biol. 2023 May 24;11:1205590. doi: 10.3389/fcell.2023.1205590. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37293126 Free PMC article. Review.
-
Preventing autoimmunity by regulating regulatory T-cell induction.J Mol Med (Berl). 2009 Dec;87(12):1153-6. doi: 10.1007/s00109-009-0536-2. J Mol Med (Berl). 2009. PMID: 19784611 Review. No abstract available.
Cited by
-
RAPID COMMUNICATION: TLR4 expressed but with reduced functionality on equine B lymphocytes.J Anim Sci. 2019 Apr 29;97(5):2175-2180. doi: 10.1093/jas/skz074. J Anim Sci. 2019. PMID: 30901382 Free PMC article.
-
Potentials of bone marrow cells-derived from naïve or diabetic mice in autoimmune type 1 diabetes: immunomodulatory, anti-inflammatory, anti hyperglycemic, and antioxidative.Endocrine. 2024 Dec;86(3):959-979. doi: 10.1007/s12020-024-03929-7. Epub 2024 Jul 17. Endocrine. 2024. PMID: 39014283 Free PMC article.
-
Roles of IFN-γ in tumor progression and regression: a review.Biomark Res. 2020 Sep 29;8:49. doi: 10.1186/s40364-020-00228-x. eCollection 2020. Biomark Res. 2020. PMID: 33005420 Free PMC article. Review.
-
GABA and Combined GABA with GAD65-Alum Treatment Alters Th1 Cytokine Responses of PBMCs from Children with Recent-Onset Type 1 Diabetes.Biomedicines. 2023 Jul 10;11(7):1948. doi: 10.3390/biomedicines11071948. Biomedicines. 2023. PMID: 37509587 Free PMC article.
-
Biomaterials-based nanoparticles conjugated to regulatory T cells provide a modular system for localized delivery of pharmacotherapeutic agents.J Biomed Mater Res A. 2023 Feb;111(2):185-197. doi: 10.1002/jbm.a.37442. Epub 2022 Sep 9. J Biomed Mater Res A. 2023. PMID: 36082558 Free PMC article.
References
-
- El-Sheikh A, Suarez-Pinzon WL, Power RF, Rabinovitch A. Both CD4(+) and CD8(+) T cells are required for IFN-gamma gene expression in pancreatic islets and autoimmune diabetes development in biobreeding rats. J Autoimmun 1999;12:109–119 - PubMed
-
- Schloot NC, Hanifi-Moghaddam P, Goebel C, et al. . Serum IFN-gamma and IL-10 levels are associated with disease progression in non-obese diabetic mice. Diabetes Metab Res Rev 2002;18:64–70 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

