Toll-Like Receptor 9 Stimulation Induces Aberrant Expression of a Proliferation-Inducing Ligand by Tonsillar Germinal Center B Cells in IgA Nephropathy
- PMID: 27920152
- PMCID: PMC5373453
- DOI: 10.1681/ASN.2016050496
Toll-Like Receptor 9 Stimulation Induces Aberrant Expression of a Proliferation-Inducing Ligand by Tonsillar Germinal Center B Cells in IgA Nephropathy
Abstract
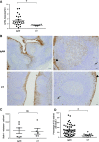
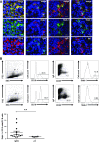
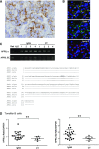
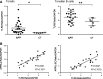
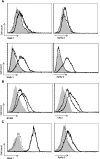
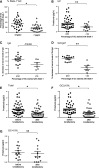
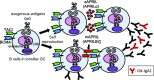
The TNF family member a proliferation-inducing ligand (APRIL; also known as TNFSF13), produced by myeloid cells, participates in the generation and survival of antibody-producing plasma cells. We studied the potential role of APRIL in the pathogenesis of IgA nephropathy (IgAN). We found that a significant proportion of germinal centers (GCs) in tonsils of patients with IgAN contained cells aberrantly producing APRIL, contributing to an overall upregulation of tonsillar APRIL expression compared with that in tonsils of control patients with tonsillitis. In IgAN GC, antigen-experienced IgD-CD38+/-CD19+ B cells expressing a switched IgG/IgA B cell receptor produced APRIL. Notably, these GC B cells expressed mRNA encoding the common cleavable APRIL-α but also, the less frequent APRIL-δ/ζ mRNA, which encodes a protein that lacks a furin cleavage site and is, thus, the uncleavable membrane-bound form. Significant correlation between TLR9 and APRIL expression levels existed in tonsils from patients with IgAN. In vitro, repeated TLR9 stimulation induced APRIL expression in tonsillar B cells from control patients with tonsillitis. Clinically, aberrant APRIL expression in tonsillar GC correlated with greater proteinuria, and patients with IgAN and aberrant APRIL overexpression in tonsillar GC responded well to tonsillectomy, with parallel decreases in serum levels of galactose-deficient IgA1. Taken together, our data indicate that antibody disorders in IgAN associate with TLR9-induced aberrant expression of APRIL in tonsillar GC B cells.
Keywords: IgA nephropathy; cytokines; immunology.
Copyright © 2017 by the American Society of Nephrology.
Figures
Comment in
-
Glomerular disease: Role of tonsillar B cells in IgAN.Nat Rev Nephrol. 2017 Feb;13(2):63. doi: 10.1038/nrneph.2016.185. Epub 2016 Dec 19. Nat Rev Nephrol. 2017. PMID: 27990017 No abstract available.
References
-
- Barratt J, Feehally J: IgA nephropathy. J Am Soc Nephrol 16: 2088–2097, 2005 - PubMed
-
- Appel GB, Waldman M: The IgA nephropathy treatment dilemma. Kidney Int 69: 1939–1944, 2006 - PubMed
-
- Suzuki Y, Suzuki H, Sato D, Kajiyama T, Okazaki K, Hashimoto A, Kihara M, Yamaji K, Satake K, Nakata J, Aizawa M, Novak J, Tomino Y: Reevaluation of the mucosa-bone marrow axis in IgA nephropathy with animal models. Adv Otorhinolaryngol 72: 64–67, 2011 - PubMed
-
- Suzuki Y, Tomino Y: Potential immunopathogenic role of the mucosa-bone marrow axis in IgA nephropathy: Insights from animal models. Semin Nephrol 28: 66–77, 2008 - PubMed
-
- Kokubo T, Hiki Y, Iwase H, Horii A, Tanaka A, Nishikido J, Hotta K, Kobayashi Y: Evidence for involvement of IgA1 hinge glycopeptide in the IgA1-IgA1 interaction in IgA nephropathy. J Am Soc Nephrol 8: 915–919, 1997 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

