The Translational Significance of the Neurovascular Unit
- PMID: 27920202
- PMCID: PMC5247651
- DOI: 10.1074/jbc.R116.760215
The Translational Significance of the Neurovascular Unit
Abstract
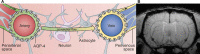
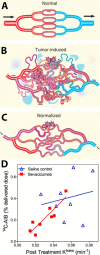
The mammalian brain is supplied with blood by specialized vasculature that is structurally and functionally distinct from that of the periphery. A defining feature of this vasculature is a physical blood-brain barrier (BBB). The BBB separates blood components from the brain microenvironment, regulating the entry and exit of ions, nutrients, macromolecules, and energy metabolites. Over the last two decades, physiological studies of cerebral blood flow dynamics have demonstrated that substantial intercellular communication occurs between cells of the vasculature and the neurons and glia that abut the vasculature. These findings suggest that the BBB does not function independently, but as a module within the greater context of a multicellular neurovascular unit (NVU) that includes neurons, astrocytes, pericytes, and microglia as well as the blood vessels themselves. Here, we describe the roles of these NVU components as well as how they act in concert to modify cerebrovascular function and permeability in health and in select diseases.
Keywords: blood-brain barrier; brain; brain tumor; cerebrovasculature; glioblastoma; neurovascular unit; vascular endothelial growth factor (VEGF); vasculogenesis.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Iadecola C. (2004) Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat. Rev. Neurosci. 5, 347–360 - PubMed
-
- Betz A. L., Firth J. A., and Goldstein G. W. (1980) Polarity of the blood-brain barrier: distribution of enzymes between the luminal and antiluminal membranes of brain capillary endothelial cells. Brain Res. 192, 17–28 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

