The MLKL Channel in Necroptosis Is an Octamer Formed by Tetramers in a Dyadic Process
- PMID: 27920255
- PMCID: PMC5311246
- DOI: 10.1128/MCB.00497-16
The MLKL Channel in Necroptosis Is an Octamer Formed by Tetramers in a Dyadic Process
Abstract
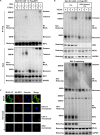
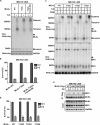
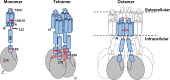
Oligomerization of the mixed-lineage kinase domain-like protein (MLKL) is essential for its cation channel function in necroptosis. Here we show that the MLKL channel is an octamer comprising two previously identified tetramers most likely in their side-by-side position. Intermolecule disulfide bonds are present in the tetramer but are not required for octamer assembly and necroptosis. MLKL forms oligomers in the necrosome and is then released from the necrosome before or during its membrane translocation. We identified two MLKL mutants that could not oligomerize into octamers, although they formed a tetramer, and also, one MLKL mutant could spontaneously form a disulfide bond-linked octamer. Subsequent analysis revealed that the tetramers fail to translocate to the plasma membrane and that the MLKL octamer formation depends on α-helices 4 and 5. While MLKL could be detected from outside the cells, its N- and C-terminal ends could not be detected, indicating that the MLKL octamer spans across the plasma membrane, leaving its N and C termini inside the cell. These data allowed us to propose a 180° symmetry model of the MLKL octamer and conclude that the fully assembled MLKL octamers, but not the previously described tetramers, act as effectors of necroptosis.
Keywords: MLKL; channel; necroptosis; octamer.
Copyright © 2017 American Society for Microbiology.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
