NMDA receptor encephalitis and other antibody-mediated disorders of the synapse: The 2016 Cotzias Lecture
- PMID: 27920282
- PMCID: PMC5177671
- DOI: 10.1212/WNL.0000000000003414
NMDA receptor encephalitis and other antibody-mediated disorders of the synapse: The 2016 Cotzias Lecture
Abstract
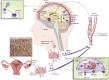
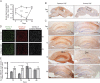
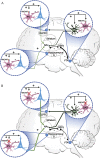
Investigations during the last 10 years have revealed a group of disorders mediated by antibodies against ion channels and synaptic receptors, which cause both neurologic and psychiatric symptoms. In this review, I discuss the process of discovery and immunologic triggers of these disorders, and use anti-NMDA receptor encephalitis to emphasize the importance of understanding the underlying physiopathologic mechanisms in those diseases. A better knowledge of these mechanisms reveals points of convergence with other disorders (e.g., schizophrenia), suggests treatment strategies beyond immunotherapy, and is helping us understand how memories are formed and retrieved.
© 2016 American Academy of Neurology.
Figures


References
-
- Drachman DB, Adams RN, Josifek LF, Self SG. Functional activities of autoantibodies to acetylcholine receptors and the clinical severity of myasthenia gravis. N Engl J Med 1982;307:769–775. - PubMed
-
- Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med 2003;349:1543–1554. - PubMed
-
- Vora NM, Holman RC, Mehal JM, Steiner CA, Blanton J, Sejvar J. Burden of encephalitis-associated hospitalizations in the United States, 1998-2010. Neurology 2014;82:443–451. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
