Feasibility of sequential adjuvant chemotherapy with a 3-month oxaliplatin-based regimen followed by 3 months of capecitabine in patients with stage III and high-risk stage II colorectal cancer: JSWOG-C2 study
- PMID: 27920498
- PMCID: PMC5125792
- DOI: 10.2147/DDDT.S112322
Feasibility of sequential adjuvant chemotherapy with a 3-month oxaliplatin-based regimen followed by 3 months of capecitabine in patients with stage III and high-risk stage II colorectal cancer: JSWOG-C2 study
Abstract
Background: Six months of oxaliplatin-based chemotherapy is the standard adjuvant chemotherapy for completely resected stage III colorectal cancer (CRC). Also, patients with stage II CRC who are considered to be at high risk of disease recurrence often receive the same adjuvant chemotherapy treatment. We prospectively investigated the extent and degree of neuropathy suffered by stage III and high-risk stage II resectable CRC patients who underwent sequential approach involving 3 months of an oxaliplatin-based regimen followed by 3 months of capecitabine.
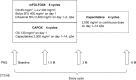
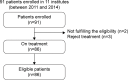
Patients and methods: Patients with completely resected stage III and high-risk stage II CRC aged ≥20 years were eligible. Patients were treated with folinic acid, fluorouracil, and oxaliplatin (FOLFOX) or capecitabine and oxaliplatin (CAPOX) for 3 months followed by capecitabine (2,500 mg/m2 on days 1-14 every 3 weeks) for 3 months. Primary end points were frequency and the grade of oxaliplatin-induced neurotoxicity as evaluated using the physician-based Common Terminology Criteria for Adverse Events version 4.0 (CTCAE) grading and the patient-based scale, self-reported Patient Neurotoxicity Questionnaire.
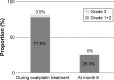
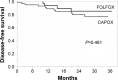
Results: Ninety-one patients were enrolled and 86 patients assessed. Eighty-four percent of patients completed the planned oxaliplatin-based therapy for 3 months, and 63% of patients completed all treatments for the full 6 months. Overall incidences of grade 3 or 4 peripheral sensory or motor neuropathy according to the CTCAE were 3.5% and 1.2%, respectively. Regarding the peripheral sensory neuropathy, the proportion of Patient Neurotoxicity Questionnaire (grade C-E) and CTCAE (grade 2-4) at months 1.5/3/6 were 11.3/22.1/29.4% and 5.3/4.4/11.3%, respectively (Spearman correlation coefficient: 0.47).
Conclusion: A sequential approach to adjuvant chemotherapy with 3 months of an oxaliplatin-based regimen followed by 3 months of capecitabine was tolerated by patients and associated with a low incidence of neuropathy.
Keywords: Patient Neurotoxicity Questionnaire; adjuvant chemotherapy; oxaliplatin; peripheral neurotoxicity.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures



Similar articles
-
Assessment of Duration and Effects of 3 vs 6 Months of Adjuvant Chemotherapy in High-Risk Stage II Colorectal Cancer: A Subgroup Analysis of the TOSCA Randomized Clinical Trial.JAMA Oncol. 2020 Apr 1;6(4):547-551. doi: 10.1001/jamaoncol.2019.6486. JAMA Oncol. 2020. PMID: 32053133 Free PMC article. Clinical Trial.
-
Phase III randomized, placebo-controlled, double-blind study of monosialotetrahexosylganglioside for the prevention of oxaliplatin-induced peripheral neurotoxicity in stage II/III colorectal cancer.Cancer Med. 2020 Jan;9(1):151-159. doi: 10.1002/cam4.2693. Epub 2019 Nov 13. Cancer Med. 2020. PMID: 31724334 Free PMC article. Clinical Trial.
-
Evaluation of FOLFOX or CAPOX reintroduction with or without bevacizumab in relapsed colorectal cancer patients treated with oxaliplatin as adjuvant chemotherapy (REACT study).Int J Clin Oncol. 2020 Aug;25(8):1515-1522. doi: 10.1007/s10147-020-01701-1. Epub 2020 May 14. Int J Clin Oncol. 2020. PMID: 32409917 Clinical Trial.
-
Clinical management of oxaliplatin-associated neurotoxicity.Clin Colorectal Cancer. 2005 Apr;5 Suppl 1:S38-46. doi: 10.3816/ccc.2005.s.006. Clin Colorectal Cancer. 2005. PMID: 15871765 Review.
-
Capecitabine plus oxaliplatin for the treatment of colorectal cancer.Expert Rev Anticancer Ther. 2008 Feb;8(2):161-74. doi: 10.1586/14737140.8.2.161. Expert Rev Anticancer Ther. 2008. PMID: 18279056 Review.
Cited by
-
Feasibility Study of a Modified XELOX Adjuvant Chemotherapy for High-Recurrence Risk Patients With Operated Stage III Colon Cancer.Front Pharmacol. 2020 Sep 18;11:583091. doi: 10.3389/fphar.2020.583091. eCollection 2020. Front Pharmacol. 2020. PMID: 33071795 Free PMC article.
-
Motor disorders related to oxaliplatin-induced peripheral neuropathy: long-term severity and impact on quality of life.Support Care Cancer. 2024 Jun 13;32(7):427. doi: 10.1007/s00520-024-08627-8. Support Care Cancer. 2024. PMID: 38869647
-
Systematic review of long-term chemotherapy-induced peripheral neuropathy (CIPN) following adjuvant oxaliplatin for colorectal cancer.Support Care Cancer. 2022 Jan;30(1):33-47. doi: 10.1007/s00520-021-06502-4. Epub 2021 Aug 19. Support Care Cancer. 2022. PMID: 34410459
-
Monosialotetrahexosylganglioside in the treatment of chronic oxaliplatin-induced peripheral neurotoxicity: TJMUCH-GI-001, a randomised controlled trial.EClinicalMedicine. 2021 Oct 29;41:101157. doi: 10.1016/j.eclinm.2021.101157. eCollection 2021 Nov. EClinicalMedicine. 2021. PMID: 34765950 Free PMC article.
-
Construction of a new clinical staging system for colorectal cancer based on the lymph node ratio: A validation study.Front Surg. 2022 Aug 25;9:929576. doi: 10.3389/fsurg.2022.929576. eCollection 2022. Front Surg. 2022. PMID: 36090338 Free PMC article.
References
-
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. - PubMed
-
- Penna C, Nordlinger B. Colorectal metastasis (liver and lung) Surg Clin North Am. 2002;82(5):1075–1090. - PubMed
-
- National Comprehensive Cancer Network . Clinical Practice Guidelines in Oncology, Colon Cancer version 2. 2016.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

