Adverse events and patients' perceived health-related quality of life at the end of multidrug-resistant tuberculosis treatment in Namibia
- PMID: 27920503
- PMCID: PMC5125758
- DOI: 10.2147/PPA.S116860
Adverse events and patients' perceived health-related quality of life at the end of multidrug-resistant tuberculosis treatment in Namibia
Abstract
Purpose: The health-related quality of life (HRQoL) of patients completing multidrug-resistant tuberculosis (MDR-TB) treatment in Namibia and whether the occurrence of adverse events influenced patients' rating of their HRQoL was evaluated.
Patients and methods: A cross-sectional analytic survey of patients completing or who recently completed MDR-TB treatment was conducted. The patients rated their HRQoL using the simplified Short Form-™ (SF-8) questionnaire consisting of eight Likert-type questions. Three supplemental questions on the adverse events that the patients may have experienced during their MDR-TB treatment were also included. Scoring of HRQoL ratings was norm-based (mean =50, standard deviation =10) ranging from 20 (worst health) to 80 (best health), rather than the conventional 0-100 scores. We evaluated the internal consistency of the scale items using the Cronbach's alpha, performed descriptive analyses, and analyzed the association between the patients' HRQoL scores and adverse events.
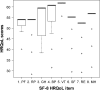
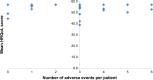
Results: Overall, 36 patients (20 males, 56%) aged 17-54 years (median =40 years) responded to the questionnaire. The median (range) HRQoL score for the physical component summary was 58.6 (35.3-60.5), while the median score for the mental component summary was 59.3 (26.6-61.9), indicating not-so-high self-rating of health. There was good internal consistency of the scale scores, with a Cronbach's alpha value of >0.80. In all, 32 (89%) of the 36 patients experienced at least one adverse drug event of any severity during their treatment (median events =3, range 1-6), of which none was life-threatening. The occurrence of adverse events was not related to HRQoL scores. For patients reporting zero to two events, the median (range) HRQoL score was 56.8 (44.4-56.8), while for those reporting three or more events, the median score was 55.2 (38.6-56.8); P=0.34 for difference between these scores.
Conclusion: Patients completing treatment for MDR-TB in Namibia tended to score moderately low on their HRQoL, using the generic SF-8 questionnaire. The occurrence of adverse events did not lead to lower HRQoL scores upon treatment completion.
Keywords: Namibia; SF-8™ questionnaire; drug safety; patient-reported health outcomes; second-line tuberculosis drugs.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

Similar articles
-
The impact of adverse events on health-related quality of life among patients receiving treatment for drug-resistant tuberculosis in Johannesburg, South Africa.Health Qual Life Outcomes. 2019 May 31;17(1):94. doi: 10.1186/s12955-019-1155-4. Health Qual Life Outcomes. 2019. PMID: 31151398 Free PMC article.
-
Health-related quality of life of patients with multidrug-resistant tuberculosis in Yemen: prospective study.Health Qual Life Outcomes. 2019 Aug 16;17(1):142. doi: 10.1186/s12955-019-1211-0. Health Qual Life Outcomes. 2019. PMID: 31420045 Free PMC article.
-
Effects of Multidrug Resistant Tuberculosis Treatment on Patients' Health Related Quality of Life: Results from a Follow Up Study.PLoS One. 2016 Jul 28;11(7):e0159560. doi: 10.1371/journal.pone.0159560. eCollection 2016. PLoS One. 2016. PMID: 27467560 Free PMC article.
-
Health-Related Quality of Life in Tuberculosis Patients in Eritrea: Comparison Among Drug-Susceptible and Rifampicin/Multidrug-Resistant Tuberculosis Patients.Patient Relat Outcome Meas. 2021 Jun 29;12:205-212. doi: 10.2147/PROM.S316337. eCollection 2021. Patient Relat Outcome Meas. 2021. PMID: 34234605 Free PMC article.
-
Health-Related Quality of Life (HRQoL) of Patients with Tuberculosis: A Review.Infect Dis Rep. 2022 Jul 18;14(4):509-524. doi: 10.3390/idr14040055. Infect Dis Rep. 2022. PMID: 35893474 Free PMC article. Review.
Cited by
-
Between Curing and Torturing: Burden of Adverse Reaction in Drug-Resistant Tuberculosis Therapy.Patient Prefer Adherence. 2021 Nov 23;15:2597-2607. doi: 10.2147/PPA.S333111. eCollection 2021. Patient Prefer Adherence. 2021. PMID: 34848950 Free PMC article. Review.
-
Impact of adjuvant therapeutic surgery on the health-related quality of life of pulmonary tuberculosis patients.ERJ Open Res. 2020 Aug 31;6(3):00083-2020. doi: 10.1183/23120541.00083-2020. eCollection 2020 Jul. ERJ Open Res. 2020. PMID: 32904577 Free PMC article.
-
Quality of Life Gain Following Treatment Among Breast Cancer Survivors With and Without HIV.JCO Glob Oncol. 2024 Aug;10:e2400110. doi: 10.1200/GO.24.00110. JCO Glob Oncol. 2024. PMID: 39116360 Free PMC article.
-
Tuberculosis patients are physically challenged and socially isolated: A mixed methods case-control study of Health Related Quality of Life in Eastern Ethiopia.PLoS One. 2018 Oct 15;13(10):e0204697. doi: 10.1371/journal.pone.0204697. eCollection 2018. PLoS One. 2018. PMID: 30321189 Free PMC article.
-
Perceptions of engagement in health care among patients with tuberculosis: a qualitative study.Patient Prefer Adherence. 2019 Jan 11;13:107-117. doi: 10.2147/PPA.S191800. eCollection 2019. Patient Prefer Adherence. 2019. PMID: 30666094 Free PMC article.
References
-
- World Health Organization (WHO) Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis. Geneva, Switzerland: World Health Organization; 2008.
-
- Sturdy A, Goodman A, José RJ, et al. Multidrug-resistant tuberculosis (MDR-TB) treatment in the UK: a study of injectable use and toxicity in practice. J Antimicrob Chemother. 2011;66(8):1815–1820. - PubMed
-
- Nathanson E, Gupta R, Huamani P, et al. Adverse events in the treatment of multidrug-resistant tuberculosis: results from the DOTS-Plus initiative. Int J Tuberc Lung Dis. 2004;8(11):1382–1384. - PubMed
-
- Shin SS, Pasechnikov AD, Gelmanova IY, et al. Adverse reactions among patients being treated for MDR-TB in Tomsk, Russia. Int J Tuberc Lung Dis. 2007;11(12):1314–1320. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

