The burden of chronic obstructive pulmonary disease associated with maintenance monotherapy in the UK
- PMID: 27920512
- PMCID: PMC5125989
- DOI: 10.2147/COPD.S109707
The burden of chronic obstructive pulmonary disease associated with maintenance monotherapy in the UK
Abstract
Background: This study characterized a cohort of chronic obstructive pulmonary disease (COPD) patients on maintenance bronchodilator monotherapy for ≥6 months to establish their disease burden, measured by health care utilization.
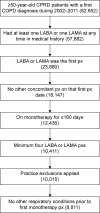
Methods: Data were extracted from the UK Clinical Practice Research Datalink and linked to Hospital Episode Statistics. The monotherapy period spanned the first prescription of a long-acting β2-adrenergic agonist or a long-acting muscarinic antagonist until the end of the study (December 31, 2013) or until step up to dual/triple therapy, for example, addition of another long-acting bronchodilator, an inhaled corticosteroid, or both. A minimum of four consecutive prescriptions and 6 months on continuous monotherapy were required. Patients <50 years old at first COPD diagnosis or with another significant respiratory disease before starting monotherapy were excluded. Disease burden was evaluated by measuring patients' rate of face-to-face interactions with a health care professional (HCP), COPD-related exacerbations, hospitalizations, and referrals.
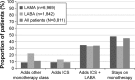
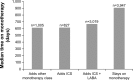
Results: A cohort of 8,811 COPD patients (95% Global initiative for chronic Obstructive Lung Disease stage A/B) on maintenance monotherapy was identified between 2002 and 2013; 45% of these patients were still on monotherapy by the end of the study. Median time from first COPD diagnosis to first monotherapy prescription was 56 days, while the median time on maintenance bronchodilator monotherapy was 2 years. The median number of prescriptions was 14. On average, patients had 15 HCP interactions per year, and one in ten patients experienced a COPD exacerbation (N=8,811). One in 50 patients were hospitalized for COPD per year (n=4,848).
Conclusion: The average monotherapy-treated patient had a higher than average HCP interaction rate. We also identified a large cohort of patients who were stepped up to triple therapy despite a low rate of exacerbations. The use of the new class of long-acting muscarinic antagonist/long-acting β2-adrenergic agonist fixed-dose combinations may provide a useful step-up treatment option in such monotherapy patients, before the use of inhaled corticosteroids.
Keywords: COPD; UK primary care setting; bronchodilators; monotherapy; prescribing patterns.
Conflict of interest statement
SE and SF are former employees of Boehringer Ingelheim Ltd., and AS, GC, and AT are currently employed by Boehringer Ingelheim Ltd. BJL has received payments for consulting and advisory boards from BI, Chiesi, Cipla, Dr Reddys, Sandoz, and Teva; support to attend educational meetings from BI, Teva, and Chiesi; and payment for talks from Meda and Teva. The Scottish Centre for Respiratory Research has received unrestricted research grant support from Teva, Chiesi, and Almirral and payment for multicenter trials from AstraZeneca, Janssen, Teva, and Roche. The authors report no other conflicts of interest in this work.
Figures

Similar articles
-
Factors influencing treatment escalation from long-acting muscarinic antagonist monotherapy to triple therapy in patients with COPD: a retrospective THIN-database analysis.Int J Chron Obstruct Pulmon Dis. 2018 Mar 5;13:781-792. doi: 10.2147/COPD.S153655. eCollection 2018. Int J Chron Obstruct Pulmon Dis. 2018. PMID: 29551894 Free PMC article.
-
The reasons for triple therapy in stable COPD patients in Japanese clinical practice.Int J Chron Obstruct Pulmon Dis. 2015 Jun 4;10:1053-9. doi: 10.2147/COPD.S79864. eCollection 2015. Int J Chron Obstruct Pulmon Dis. 2015. PMID: 26082629 Free PMC article.
-
An evaluation of early medication use for COPD: a population-based cohort study.Int J Chron Obstruct Pulmon Dis. 2016 Dec 7;11:3101-3108. doi: 10.2147/COPD.S123643. eCollection 2016. Int J Chron Obstruct Pulmon Dis. 2016. PMID: 27994449 Free PMC article.
-
Single-inhaler triple therapy in patients with chronic obstructive pulmonary disease: a systematic review.Respir Res. 2019 Nov 4;20(1):242. doi: 10.1186/s12931-019-1213-9. Respir Res. 2019. PMID: 31684965 Free PMC article.
-
When is dual bronchodilation indicated in COPD?Int J Chron Obstruct Pulmon Dis. 2017 Aug 3;12:2291-2305. doi: 10.2147/COPD.S138554. eCollection 2017. Int J Chron Obstruct Pulmon Dis. 2017. PMID: 28814857 Free PMC article. Review.
Cited by
-
Patient-Reported Burden of Illness in a Prevalent COPD Population Treated with Long-Acting Muscarinic Antagonist Monotherapy: A Claims-Linked Patient Survey Study.Pulm Ther. 2019 Jun;5(1):69-80. doi: 10.1007/s41030-019-0091-0. Epub 2019 Apr 1. Pulm Ther. 2019. PMID: 32026428 Free PMC article.
-
Use of medicines and health services for chronic obstructive pulmonary disease among a cohort of Australians over 50 years.Int J Chron Obstruct Pulmon Dis. 2018 Oct 4;13:3085-3093. doi: 10.2147/COPD.S172495. eCollection 2018. Int J Chron Obstruct Pulmon Dis. 2018. PMID: 30323579 Free PMC article.
References
-
- HSE Chronic obstructive pulmonary disease (COPD) in Great Britain in 2014. 2014. [Accessed February 5, 2016]. Available from: http://www.hse.gov.uk/statistics/causdis/copd/index.htm.
-
- NICE . Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care. London: National Clinical Guideline Centre – acute and chronic conditions; 2010. [Accessed February 5, 2016]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK65039/
-
- WHO Chronic obstructive pulmonary disease (COPD) [Accessed February 5, 2016];Fact Sheet. 2015 315 Available from: http://www.who.int/mediacentre/factsheets/fs315/en/
-
- Healthcare Commission . Clearing the Air: a National Study of Chronic Obstructive Pulmonary Disease. Great Britain: Commission for Healthcare Audit and Inspection; 2006.
-
- Pascoe S, Locantore N, Dransfield MT, Barnes NC, Pavord ID. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med. 2015;3(6):435–442. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

