Effect of ELOM-080 on exacerbations and symptoms in COPD patients with a chronic bronchitis phenotype - a post-hoc analysis of a randomized, double-blind, placebo-controlled clinical trial
- PMID: 27920515
- PMCID: PMC5125994
- DOI: 10.2147/COPD.S117652
Effect of ELOM-080 on exacerbations and symptoms in COPD patients with a chronic bronchitis phenotype - a post-hoc analysis of a randomized, double-blind, placebo-controlled clinical trial
Abstract
Background: Treating symptoms and preventing exacerbations are key components of chronic obstructive pulmonary disease (COPD) long-term management. Recently, a more tailored treatment approach has been proposed, in particular for two well-established clinical phenotypes, frequent exacerbators and chronic bronchitis-dominant COPD. ELOM-080 has demonstrated clinical efficacy in treating symptoms and preventing exacerbations in subjects with chronic bronchitis. However, little is known about the potential effects of ELOM-080 in COPD patients.
Aim: To evaluate the effect on exacerbation, cough sputum, and general state of health of long-term treatment with ELOM-080 in COPD patients with an exacerbation history and chronic bronchitis.
Methods: We performed a post-hoc analysis of a randomized, double-blinded, placebo-controlled parallel-group clinical trial of a 6-month treatment with ELOM-080 (3×300 mg) in patients with chronic bronchitis and concomitant COPD. The primary outcome was the proportion of subjects with at least one exacerbation over the 6-month study period. Secondary outcomes included the total number of exacerbations (ie, cumulative occurrence of exacerbations during the study period) and the proportion of acute exacerbations necessitating an antibiotic treatment, monthly evaluations of sputum and cough symptoms, and the general state of health and a safety analysis.
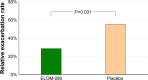
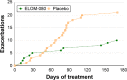
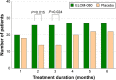
Results: Of 260 randomized subjects, 64 patients fulfilled the inclusion criteria for COPD (ELOM-080: 35, placebo: 29). Compared to placebo, ELOM-080 reduced the percentage of subjects with at least one exacerbation (29% versus 55%, P=0.031) and a reduction in the overall occurrence of exacerbations (ELOM-080: 10, placebo: 21, P=0.012) during the winter season. The percentage of asymptomatic or mildly symptomatic patients (sputum/expectoration and cough) was consistently higher in the ELOM-080 group compared to placebo, with statistical significant differences after 2 and 3 months of treatment (2 months: ELOM-080 25%, placebo 11%, P<0.005; 3 months: ELOM-080 26%, placebo 14%, P<0.05). Likewise the subjective rating of general health status was better in the ELOM-080 group with statistically significant superiority after 2 and 3 months of treatment (2-month treatment: P=0.015; 3-month treatment: P=0.024). Tolerability results were comparable between ELOM-080 and placebo.
Conclusion: ELOM-080 is efficacious in patients with COPD and a chronic bronchitis phenotype. Prophylactic use reduces the rate of exacerbations and improves the key symptoms of sputum and cough with a favorable long-term tolerability profile.
Keywords: COPD; chronic bronchitis; exacerbations; myrtol; phytotherapy; sputum; winter.
Conflict of interest statement
The institution where KMB and JB are employed has received lecture fees, advisory honoraria, and compensation for design and conduct of clinical trials from various pharmaceutical companies in the past 5 years, including honoraria from G. Pohl-Boskamp GmbH&Co KG. HC and TW are full-time employees of G. Pohl-Boskamp GmbH & Co KG. The authors report no other conflicts of interest in this work.
Figures


References
-
- Decramer M. GOLD: Global strategy for the diagnosis, management, and prevention of COPD. 2016. [Updated, 2016]. Available from: www.goldcopd.com.
-
- Rabe KF, Fabbri LM, Vogelmeier C, et al. Seasonal distribution of COPD exacerbations in the prevention of exacerbations with tiotropium in COPD trial. Chest. 2013;143(3):711–719. - PubMed
-
- Hurst JR, Donaldson GC, Quint JK, Goldring JJ, Baghai-Ravary R, Wedzicha JA. Temporal clustering of exacerbations in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;179(5):369–374. - PubMed
-
- Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

