Extending a Single Residue Switch for Abbreviating Catalysis in Plant ent-Kaurene Synthases
- PMID: 27920791
- PMCID: PMC5118566
- DOI: 10.3389/fpls.2016.01765
Extending a Single Residue Switch for Abbreviating Catalysis in Plant ent-Kaurene Synthases
Abstract
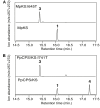
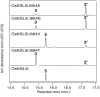
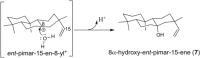
Production of ent-kaurene as a precursor for important signaling molecules such as the gibberellins seems to have arisen early in plant evolution, with corresponding cyclase(s) present in all land plants (i.e., embryophyta). The relevant enzymes seem to represent fusion of the class II diterpene cyclase that produces the intermediate ent-copalyl diphosphate (ent-CPP) and the subsequently acting class I diterpene synthase that produces ent-kaurene, although the bifunctionality of the ancestral gene is only retained in certain early diverging plants, with gene duplication and sub-functionalization leading to distinct ent-CPP synthases and ent-kaurene synthases (KSs) generally observed. This evolutionary scenario implies that plant KSs should have conserved structural features uniquely required for production of ent-kaurene relative to related enzymes that have alternative function. Notably, substitution of threonine for a conserved isoleucine has been shown to "short-circuit" the complex bicyclization and rearrangement reaction catalyzed by KSs after initial cyclization, leading to predominant production of ent-pimaradiene, at least in KSs from angiosperms. Here this effect is shown to extend to KSs from earlier diverging plants (i.e., bryophytes), including a bifunctional/KS. In addition, attribution of the dramatic effect of this single residue "switch" on product outcome to electrostatic stabilization of the ent-pimarenyl carbocation intermediate formed upon initial cyclization by the hydroxyl introduced by threonine substitution has been called into question by the observation of similar effects from substitution of alanine. Here further mutational analysis and detailed product analysis is reported that supports the importance of electrostatic stabilization by a hydroxyl or water.
Keywords: carbocation; catalytic mechanism; diterpene synthases; diterpenoids; metabolic engineering; mutagenesis; product specificity.
Figures



Similar articles
-
Divergent Evolution of the Diterpene Biosynthesis Pathway in Tea Plants (Camellia sinensis) Caused by Single Amino Acid Variation of ent-Kaurene Synthase.J Agric Food Chem. 2020 Sep 16;68(37):9930-9939. doi: 10.1021/acs.jafc.0c03488. Epub 2020 Sep 3. J Agric Food Chem. 2020. PMID: 32841021
-
One amino acid makes the difference: the formation of ent-kaurene and 16α-hydroxy-ent-kaurane by diterpene synthases in poplar.BMC Plant Biol. 2015 Oct 28;15:262. doi: 10.1186/s12870-015-0647-6. BMC Plant Biol. 2015. PMID: 26511849 Free PMC article.
-
Gibberellin-biosynthetic ent-kaurene synthases in higher plants do not require their non-catalytic domains for the catalysis.Biochem J. 2024 Jun 19;481(12):779-791. doi: 10.1042/BCJ20240162. Biochem J. 2024. PMID: 38829839
-
Evolution of Labdane-Related Diterpene Synthases in Cereals.Plant Cell Physiol. 2020 Dec 23;61(11):1850-1859. doi: 10.1093/pcp/pcaa106. Plant Cell Physiol. 2020. PMID: 32810270 Review.
-
The family of terpene synthases in plants: a mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom.Plant J. 2011 Apr;66(1):212-29. doi: 10.1111/j.1365-313X.2011.04520.x. Plant J. 2011. PMID: 21443633 Review.
Cited by
-
A Pair of Residues That Interactively Affect Diterpene Synthase Product Outcome.ACS Chem Biol. 2017 Mar 17;12(3):862-867. doi: 10.1021/acschembio.6b01075. Epub 2017 Feb 14. ACS Chem Biol. 2017. PMID: 28170228 Free PMC article.
-
Exploring the Influence of Domain Architecture on the Catalytic Function of Diterpene Synthases.Biochemistry. 2017 Apr 11;56(14):2010-2023. doi: 10.1021/acs.biochem.7b00137. Epub 2017 Mar 31. Biochemistry. 2017. PMID: 28362483 Free PMC article.
-
Engineering terpene synthases and their substrates for the biocatalytic production of terpene natural products and analogues.Chem Commun (Camb). 2025 Feb 4;61(12):2468-2483. doi: 10.1039/d4cc05785f. Chem Commun (Camb). 2025. PMID: 39784321 Free PMC article. Review.
-
Biosynthesis of the oxygenated diterpene nezukol in the medicinal plant Isodon rubescens is catalyzed by a pair of diterpene synthases.PLoS One. 2017 Apr 26;12(4):e0176507. doi: 10.1371/journal.pone.0176507. eCollection 2017. PLoS One. 2017. PMID: 28445526 Free PMC article.
-
Combinatorial biosynthesis and the basis for substrate promiscuity in class I diterpene synthases.Metab Eng. 2019 Sep;55:44-58. doi: 10.1016/j.ymben.2019.06.008. Epub 2019 Jun 17. Metab Eng. 2019. PMID: 31220664 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

