Age-Related Differences in the Luminal and Mucosa-Associated Gut Microbiome of Broiler Chickens and Shifts Associated with Campylobacter jejuni Infection
- PMID: 27921008
- PMCID: PMC5118433
- DOI: 10.3389/fcimb.2016.00154
Age-Related Differences in the Luminal and Mucosa-Associated Gut Microbiome of Broiler Chickens and Shifts Associated with Campylobacter jejuni Infection
Abstract
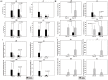
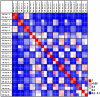
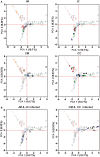
Despite the importance of gut microbiota for broiler performance and health little is known about the composition of this ecosystem, its development and response towards bacterial infections. Therefore, the current study was conducted to address the composition and structure of the microbial community in broiler chickens in a longitudinal study from day 1 to day 28 of age in the gut content and on the mucosa. Additionally, the consequences of a Campylobacter (C.) jejuni infection on the microbial community were assessed. The composition of the gut microbiota was analyzed with 16S rRNA gene targeted Illumina MiSeq sequencing. Sequencing of 130 samples yielded 51,825,306 quality-controlled sequences, which clustered into 8285 operational taxonomic units (OTUs; 0.03 distance level) representing 24 phyla. Firmicutes, Proteobacteria, Bacteroidetes, Actinobacteria, and Tenericutes were the main components of the gut microbiota, with Proteobacteria and Firmicutes being the most abundant phyla (between 95.0 and 99.7% of all sequences) at all gut sites. Microbial communities changed in an age-dependent manner. Whereas, young birds had more Proteobacteria, Firmicutes, and Tenericutes dominated in older birds (>14 days old). In addition, 28 day old birds had more diverse bacterial communities than young birds. Furthermore, numerous significant differences in microbial profiles between the mucosa and luminal content of the small and large intestine were detected, with some species being strongly associated with the mucosa whereas others remained within the luminal content of the gut. Following oral infection of 14 day old broiler chickens with 1 × 108 CFU of C. jejuni NCTC 12744, it was found that C. jejuni heavily colonized throughout the small and large intestine. Moreover, C. jejuni colonization was associated with an alteration of the gut microbiota with infected birds having a significantly lower abundance of Escherichia (E.) coli at different gut sites. On the contrary, the level of Clostridium spp. was higher in infected birds compared with birds from the negative controls. In conclusion, the obtained results demonstrate how the bacterial microbiome composition changed within the early life of broiler chickens in the gut lumen and on the mucosal surface. Furthermore, our findings confirmed that the Campylobacter carrier state in chicken is characterized by multiple changes in the intestinal ecology within the host.
Keywords: 16S rRNA gene; Campylobacter jejuni; MiSeq sequencing; age; broiler chickens; luminal content; microbiota; mucosa.
Figures




References
-
- Apajalahti J. H. A., Kettunen A., Graham H. (2004). Characteristics of the gastro-intestinal microbial communities with special reference to the chicken. World's Poult. Sci. J. 60, 223–232. 10.1079/WPS20040017 - DOI
-
- Awad W. A., Dublecz F., Hess C., Dublecz K., Khayal B., Aschenbach J. R., et al. . (2016). Campylobacter jejuni colonization promotes the translocation of Escherichia coli to extra-intestinal organs and disturbs the short-chain fatty acids profiles in the chicken gut. Poult. Sci. 95, 2259–2265. 10.3382/ps/pew151 - DOI - PubMed
-
- Awad W. A., Smorodchenko A., Hess C., Aschenbach J. R., Molnár A., Dublecz K., et al. . (2015b). Increased intracellular calcium level and impaired nutrient absorption are important pathogenicity traits in the chicken intestinal epithelium during Campylobacter jejuni colonization. Appl. Microbiol. Biotechnol. 99, 6431–6441. 10.1007/s00253-015-6543-z - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

