Blood flow controls bone vascular function and osteogenesis
- PMID: 27922003
- PMCID: PMC5150650
- DOI: 10.1038/ncomms13601
Blood flow controls bone vascular function and osteogenesis
Abstract
While blood vessels play important roles in bone homeostasis and repair, fundamental aspects of vascular function in the skeletal system remain poorly understood. Here we show that the long bone vasculature generates a peculiar flow pattern, which is important for proper angiogenesis. Intravital imaging reveals that vessel growth in murine long bone involves the extension and anastomotic fusion of endothelial buds. Impaired blood flow leads to defective angiogenesis and osteogenesis, and downregulation of Notch signalling in endothelial cells. In aged mice, skeletal blood flow and endothelial Notch activity are also reduced leading to decreased angiogenesis and osteogenesis, which is reverted by genetic reactivation of Notch. Blood flow and angiogenesis in aged mice are also enhanced on administration of bisphosphonate, a class of drugs frequently used for the treatment of osteoporosis. We propose that blood flow and endothelial Notch signalling are key factors controlling ageing processes in the skeletal system.
Figures
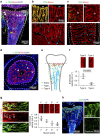


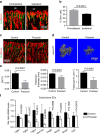
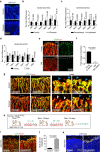


References
-
- Karsenty G. & Wagner E. F. Reaching a genetic and molecular understanding of skeletal development. Dev. Cell 2, 389–406 (2002). - PubMed
-
- Kronenberg H. M. Developmental regulation of the growth plate. Nature 423, 332–336 (2003). - PubMed
-
- Eshkar-Oren I. et al.. The forming limb skeleton serves as a signaling center for limb vasculature patterning via regulation of Vegf. Development 136, 1263–1272 (2009). - PubMed
-
- Gerber H. P. et al.. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat. Med. 5, 623–628 (1999). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

