The progeroid gene BubR1 regulates axon myelination and motor function
- PMID: 27922816
- PMCID: PMC5191862
- DOI: 10.18632/aging.101032
The progeroid gene BubR1 regulates axon myelination and motor function
Abstract
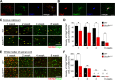
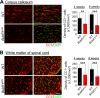
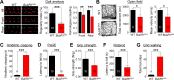
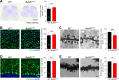
Myelination, the process by which oligodendrocytes form the myelin sheath around axons, is key to axonal signal transduction and related motor function in the central nervous system (CNS). Aging is characterized by degenerative changes in the myelin sheath, although the molecular underpinnings of normal and aberrant myelination remain incompletely understood. Here we report that axon myelination and related motor function are dependent on BubR1, a mitotic checkpoint protein that has been linked to progeroid phenotypes when expressed at low levels and healthy lifespan when overabundant. We found that oligodendrocyte progenitor cell proliferation and oligodendrocyte density is markedly reduced in mutant mice with low amounts of BubR1 (BubR1H/H mice), causing axonal hypomyelination in both brain and spinal cord. Expression of essential myelin-related genes such as MBP and PLP1 was significantly reduced in these tissues. Consistent with defective myelination, BubR1H/H mice exhibited various motor deficits, including impaired motor strength, coordination, and balance, irregular gait patterns and reduced locomotor activity. Collectively, these data suggest that BubR1 is a key determinant of oligodendrocyte production and function and provide a molecular entry point to understand age-related degenerative changes in axon myelination.
Keywords: BubR1; corpus callosum; motor function; myelination; oligodendrocytes; spinal cord.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Calvo-Ochoa E, Arias C. Cellular and metabolic alterations in the hippocampus caused by insulin signalling dysfunction and its association with cognitive impairment during aging and Alzheimer's disease: studies in animal models. Diabetes Metab Res Rev. 2015;31:1–13. doi: 10.1002/dmrr.2531. - DOI - PubMed
-
- Tucsek Z, Toth P, Sosnowska D, Gautam T, Mitschelen M, Koller A, Szalai G, Sonntag WE, Ungvari Z, Csiszar A. Obesity in aging exacerbates blood-brain barrier disruption, neuroinflammation, and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer's disease. J Gerontol A Biol Sci Med Sci. 2014;69:1212–26. doi: 10.1093/gerona/glt177. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

