Impaired energy metabolism of senescent muscle satellite cells is associated with oxidative modifications of glycolytic enzymes
- PMID: 27922824
- PMCID: PMC5270674
- DOI: 10.18632/aging.101126
Impaired energy metabolism of senescent muscle satellite cells is associated with oxidative modifications of glycolytic enzymes
Abstract
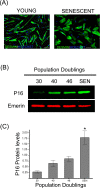
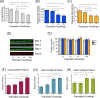
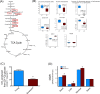
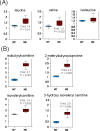
Accumulation of oxidized proteins is a hallmark of cellular and organismal aging. Adult muscle stem cell (or satellite cell) replication and differentiation is compromised with age contributing to sarcopenia. However, the molecular events related to satellite cell dysfunction during aging are not completely understood. In the present study we have addressed the potential impact of oxidatively modified proteins on the altered metabolism of senescent human satellite cells. By using a modified proteomics analysis we have found that proteins involved in protein quality control and glycolytic enzymes are the main targets of oxidation (carbonylation) and modification with advanced glycation/lipid peroxidation end products during the replicative senescence of satellite cells. Inactivation of the proteasome appeared to be a likely contributor to the accumulation of such damaged proteins. Metabolic and functional analyses revealed an impaired glucose metabolism in senescent cells. A metabolic shift leading to increased mobilization of non-carbohydrate substrates such as branched chain amino acids or long chain fatty acids was observed. Increased levels of acyl-carnitines indicated an increased turnover of storage and membrane lipids for energy production. Taken together, these results support a link between oxidative protein modifications and the altered cellular metabolism associated with the senescent phenotype of human myoblasts.
Keywords: aging; cellular senescence; energy metabolism; myoblasts; protein oxidation; proteostasis.
Conflict of interest statement
The authors have no conflict of interests to declare.
Figures


References
-
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M. and European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–23. doi: 10.1093/ageing/afq034. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

