Protocol of a prospective study for the combination treatment of Shu-Gan-jian-Pi decoction and steroid standard therapy in autoimmune hepatitis patients
- PMID: 27923369
- PMCID: PMC5142150
- DOI: 10.1186/s12906-016-1486-1
Protocol of a prospective study for the combination treatment of Shu-Gan-jian-Pi decoction and steroid standard therapy in autoimmune hepatitis patients
Abstract
Background: Prednisone plus azathioprine is considered the mainstay of therapy in the current recommendations for autoimmune hepatitis (AIH). However, it does not provide good benefits for AIH patients because of its serious side effects. Therefore, more and more AIH patients prefer to seek for traditional Chinese medicine (TCM) to manage their symptoms and reduce the side effects of steroids in China. Shu-Gan-Jian-Pi Decoction is a popular used Chinese herbal formula in Guangdong province of China, which has demonstrated the effect of improving efficacy and reducing side effects of corticosteroids in AIH patients. The aim of this study is to evaluate the effects of Shu-Gan-Jian-Pi Decoction combined with steroid in AIH patients. So, this study aims to explore whether the combination treatment of Shu-Gan-Jian-Pi Decoction and steroid standard therapy could improve the clinical management of AIH.
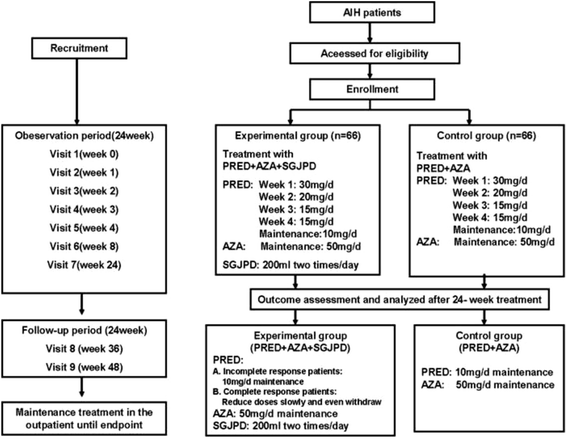
Methods: A prospective non-randomized study on AIH will be conducted between October 2015 and June 2017 in Guangdong Provincial hospital of Chinese medicine. Eligible AIH patients will be classified as the case group (n = 66) and the control group (n = 66) based on the interventions. Patients taking Shu-Gan-Jian-Pi Decoction combined with prednisone and azathioprine will be in the case group and those taking prednisone and azathioprine will be in the control group. The whole study will last 48 weeks, including a 24-week observation period and a 24-week follow-up period. The primary outcome was complete response to therapy, defined as complete biochemical remission at the patient's last visit of observation period and the absence of predefined steroid-specific side effects throughout treatment.
Discussion: This trial will evaluate the efficacy and safety of Shu-Gan-Jian-Pi Decoction combined with prednisone and azathioprine on AIH patients. The achievement of this trial will provide evidence-based data for Shu-Gan-Jian-Pi Decoction, which could provide good benefits for AIH patients.
Trial registration: Chinese Clinical Trial Registry: ChiCTR-OOC-15006155 . Registration date: 28 March 2015.
Keywords: Autoimmune Hepatitis; Shu-Gan-Jian-Pi Decoction; Side effects; Steroid.
Figures
Similar articles
-
Budesonide versus prednisone with azathioprine for the treatment of autoimmune hepatitis in children and adolescents.J Pediatr. 2013 Nov;163(5):1347-53.e1. doi: 10.1016/j.jpeds.2013.05.042. Epub 2013 Jun 28. J Pediatr. 2013. PMID: 23810723 Clinical Trial.
-
Traditional Chinese medicine combination therapy for patients with steroid-dependent ulcerative colitis: study protocol for a randomized controlled trial.Trials. 2017 Jan 10;18(1):8. doi: 10.1186/s13063-016-1763-9. Trials. 2017. PMID: 28069051 Free PMC article. Clinical Trial.
-
Azathioprine Monotherapy Is Equivalent to Dual Therapy in Maintaining Remission in Autoimmune Hepatitis.Dig Dis Sci. 2021 May;66(5):1715-1719. doi: 10.1007/s10620-020-06347-7. Epub 2020 May 20. Dig Dis Sci. 2021. PMID: 32436124
-
Therapeutic strategies for autoimmune hepatitis.Dig Dis. 2011;29(4):411-5. doi: 10.1159/000329805. Epub 2011 Aug 30. Dig Dis. 2011. PMID: 21894012 Review.
-
Autoimmune Hepatitis in the Elderly: Diagnosis and Pharmacologic Management.Drugs Aging. 2018 Jul;35(7):589-602. doi: 10.1007/s40266-018-0556-0. Drugs Aging. 2018. PMID: 29971609 Review.
Cited by
-
Guizhi Fuling Wan ameliorates concanavalin A-induced autoimmune hepatitis in mice.Biomed J. 2025 Feb;48(1):100731. doi: 10.1016/j.bj.2024.100731. Epub 2024 Apr 26. Biomed J. 2025. PMID: 38677491 Free PMC article.
-
Ginsenosides Regulates Innate Immunity to Affect Immune Microenvironment of AIH Through Hippo-YAP/TAZ Signaling Pathway.Front Immunol. 2022 Feb 11;13:851560. doi: 10.3389/fimmu.2022.851560. eCollection 2022. Front Immunol. 2022. PMID: 35222444 Free PMC article.
-
Chinese medicine in the treatment of autoimmune hepatitis: Progress and future opportunities.Animal Model Exp Med. 2022 Apr;5(2):95-107. doi: 10.1002/ame2.12201. Epub 2022 Jan 19. Animal Model Exp Med. 2022. PMID: 35263512 Free PMC article. Review.
-
Comparative efficacy and tolerability of treatments for adult autoimmune hepatitis: A systematic review and network meta-analysis.Exp Ther Med. 2018 Jun;15(6):4838-4850. doi: 10.3892/etm.2018.6063. Epub 2018 Apr 13. Exp Ther Med. 2018. PMID: 29904396 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous