Immunomodulation after ischemic stroke: potential mechanisms and implications for therapy
- PMID: 27923376
- PMCID: PMC5141640
- DOI: 10.1186/s13054-016-1573-1
Immunomodulation after ischemic stroke: potential mechanisms and implications for therapy
Erratum in
-
Erratum to: Immunomodulation after ischemic stroke: potential mechanisms and implications for therapy.Crit Care. 2017 Oct 18;21(1):256. doi: 10.1186/s13054-017-1834-7. Crit Care. 2017. PMID: 29047358 Free PMC article. No abstract available.
Abstract
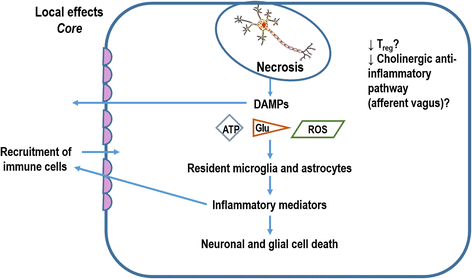
Brain injuries are often associated with intensive care admissions, and carry high morbidity and mortality rates. Ischemic stroke is one of the most frequent causes of injury to the central nervous system. It is now increasingly clear that human stroke causes multi-organ systemic disease. Brain inflammation may lead to opposing local and systemic effects. Suppression of systemic immunity by the nervous system could protect the brain from additional inflammatory damage; however, it may increase the susceptibility to infection. Pneumonia and urinary tract infection are the most common complications occurring in patients after stroke. The mechanisms involved in lung-brain interactions are still unknown, but some studies have suggested that inhibition of the cholinergic anti-inflammatory pathway and release of glucocorticoids, catecholamines, and damage-associated molecular patterns (DAMPs) are among the pathophysiological mechanisms involved in communication from the ischemic brain to the lungs after stroke. This review describes the modifications in local and systemic immunity that occur after stroke, outlines mechanisms of stroke-induced immunosuppression and their role in pneumonia, and highlights potential therapeutic targets to reduce post-stroke complications. Despite significant advances towards a better understanding of the pathophysiology of ischemic stroke-induced immunosuppression and stroke-associated pneumonia (SAP) in recent years, many unanswered questions remain. The true incidence and outcomes of SAP, especially in intensive care unit settings, have yet to be determined, as has the full extent of stroke-induced immunosuppression and its clinical implications.
Keywords: Damage-associated molecular patterns; Immunosuppression; Inflammation; Ischemic stroke; Stroke-associated pneumonia.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

