Regulation of membrane type-1 matrix metalloproteinase activity and intracellular localization in clinical thoracic aortic aneurysms
- PMID: 27923483
- PMCID: PMC5316335
- DOI: 10.1016/j.jtcvs.2016.10.065
Regulation of membrane type-1 matrix metalloproteinase activity and intracellular localization in clinical thoracic aortic aneurysms
Abstract
Objective: Membrane type-1 matrix metalloproteinase (MT1-MMP) is elevated during thoracic aortic aneurysm (TAA) development in mouse models, and plays an important role in the activation of matrix metalloproteinase (MMP)-2 and the release of matrix- bound transforming growth factor-β. In this study, we tested the hypothesis that MT1-MMP is subject to protein kinase C (PKC)-mediated regulation, which alters intracellular trafficking and activity with TAAs.
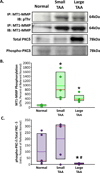
Methods: Levels of MMP-2, native and phosphorylated MT1-MMP, and PKC-δ were measured in aortic tissue from patients with small TAAs (<5 cm; n = 8) and large TAAs (>6.5 cm; n = 8), and compared with values measured in normal controls (n = 8). Cellular localization of green fluorescent protein (GFP)-tagged MT1-MMP was assessed in aortic fibroblasts isolated from control and 4-week TAA mice. The effects of PKC-mediated phosphorylation on MT1-MMP cellular localization and function (active MMP-2 vs phospo-Smad2 abundance) were assessed after treatment with a PKC activator (phorbol-12-myristate-13-acetate [PMA], 100 nM) with and without a PKC-δ-specific inhibitor (röttlerin, 3 μM).
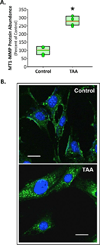
Results: Compared with controls, MT1-MMP abundance was increased in aortas from both TAA groups. Active MMP-2 was increased only in the large TAA group. The abundances of phosphorylated MT1-MMP and activated PKC-δ were enhanced in the small TAA group compared with the large TAA group. MT1-MMP was localized on the plasma membrane in aortic fibroblasts from control mice and in endosomes from TAA mice. Treatment with PMA induced MT1-MMP-GFP internalization, enhanced phospho-Smad2, and reduced MMP-2 activation, whereas röttlerin pretreatment inhibited these effects.
Conclusions: Phosphorylation of MT1-MMP mediates its activity through directing cellular localization, shifting its role from MMP-2 activation to intracellular signaling. Thus, targeted inhibition of MT1-MMP may have therapeutic relevance as an approach to attenuating TAA development.
Keywords: MT1-MMP; aneurysm; protein kinase C; remodeling; thoracic aorta.
Copyright © 2016 The American Association for Thoracic Surgery. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures



Comment in
-
The matrix reloaded: Which pill to take to attenuate thoracic aortic aneurysm development?J Thorac Cardiovasc Surg. 2017 Mar;153(3):547-548. doi: 10.1016/j.jtcvs.2016.10.072. Epub 2016 Nov 14. J Thorac Cardiovasc Surg. 2017. PMID: 27964978 No abstract available.
References
-
- Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorac Surg. 2002;74:S1877–S1880. discussion S1892-1878. - PubMed
-
- Barbour JR, Spinale FG, Ikonomidis JS. Proteinase systems and thoracic aortic aneurysm progression. J Surg Res. 2007;139:292–307. - PubMed
-
- Shen M, Lee J, Basu R, et al. Divergent roles of matrix metalloproteinase 2 in pathogenesis of thoracic aortic aneurysm. Arterioscler Thromb Vasc Biol. 2015;35:888–898. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

