IL-17 Receptor Signaling in the Lung Epithelium Is Required for Mucosal Chemokine Gradients and Pulmonary Host Defense against K. pneumoniae
- PMID: 27923703
- PMCID: PMC5149406
- DOI: 10.1016/j.chom.2016.10.003
IL-17 Receptor Signaling in the Lung Epithelium Is Required for Mucosal Chemokine Gradients and Pulmonary Host Defense against K. pneumoniae
Abstract
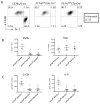
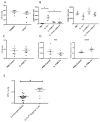
The cytokine IL-17, and signaling via its heterodimeric IL-17RA/IL-17RC receptor, is critical for host defense against extracellular bacterial and fungal pathogens. Polarized lung epithelial cells express IL-17RA and IL-17RC basolaterally. However, their contribution to IL-17-dependent pulmonary defenses in vivo remains to be determined. To address this, we generated mice with conditional deletion of Il17ra or Il17rc in Scgb1a1-expressing club cells, a major component of the murine bronchiolar epithelium. These mice displayed an impaired ability to recruit neutrophils into the airway lumen in response to IL-17, a defect in bacterial clearance upon mucosal challenge with the pulmonary pathogen Klebsiella pneumoniae, and substantially reduced epithelial expression of the chemokine Cxcl5. Neutrophil recruitment and bacterial clearance were restored by intranasal administration of recombinant CXCL5. Our data show that IL-17R signaling in the lung epithelium plays a critical role in establishing chemokine gradients that are essential for mucosal immunity against pulmonary bacterial pathogens.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

