Type of supplemented simple sugar, not merely calorie intake, determines adverse effects on metabolism and aortic function in female rats
- PMID: 27923787
- PMCID: PMC5336577
- DOI: 10.1152/ajpheart.00339.2016
Type of supplemented simple sugar, not merely calorie intake, determines adverse effects on metabolism and aortic function in female rats
Abstract
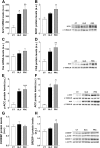
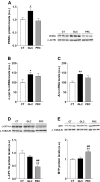
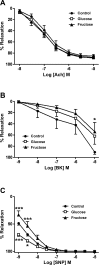
High consumption of simple sugars causes adverse cardiometabolic effects. We investigated the mechanisms underlying the metabolic and vascular effects of glucose or fructose intake and determined whether these effects are exclusively related to increased calorie consumption. Female Sprague-Dawley rats were supplemented with 20% wt/vol glucose or fructose for 2 mo, and plasma analytes and aortic response to vasodilator and vasoconstrictor agents were determined. Expression of molecules associated with lipid metabolism, insulin signaling, and vascular response were evaluated in hepatic and/or aortic tissues. Caloric intake was increased in both sugar-supplemented groups vs. control and in glucose- vs. fructose-supplemented rats. Hepatic lipogenesis was induced in both groups. Plasma triglycerides were increased only in the fructose group, together with decreased expression of carnitine palmitoyltransferase-1A and increased microsomal triglyceride transfer protein expression in the liver. Plasma adiponectin and peroxisome proliferator-activated receptor (PPAR)-α expression was increased only by glucose supplementation. Insulin signaling in liver and aorta was impaired in both sugar-supplemented groups, but the effect was more pronounced in the fructose group. Fructose supplementation attenuated aortic relaxation response to a nitric oxide (NO) donor, whereas glucose potentiated it. Phenylephrine-induced maximal contractions were reduced in the glucose group, which could be related to increased endothelial NO synthase (eNOS) phosphorylation and subsequent elevated basal NO in the glucose group. In conclusion, despite higher caloric intake in glucose-supplemented rats, fructose caused worse metabolic and vascular responses. This may be because of the elevated adiponectin level and the subsequent enhancement of PPARα and eNOS phosphorylation in glucose-supplemented rats.
New & noteworthy: This is the first study comparing the effects of glucose and fructose consumption on metabolic factors and aortic function in female rats. Our results show that, although total caloric consumption was higher in glucose-supplemented rats, fructose ingestion had a greater impact in inducing metabolic and aortic dysfunction.
Keywords: adiponectin; fructose; glucose; insulin resistance; liver.
Copyright © 2017 the American Physiological Society.
Figures





Comment in
-
Sugar-sweetened beverages and vascular function: food for thought.Am J Physiol Heart Circ Physiol. 2017 Feb 1;312(2):H285-H288. doi: 10.1152/ajpheart.00783.2016. Epub 2016 Dec 30. Am J Physiol Heart Circ Physiol. 2017. PMID: 28039204 No abstract available.
References
-
- Alegret M, Roglans N, Laguna JC. Fructose consumption and leptin resistance: what have we learnt from animal studies? In: Leptin: Hormonal Fuctions, Dysfunctions and Clinical Uses, edited by Hemling RM, Belkin AT. Hauppauge, NY: Nova Science, 2011.
-
- Aszódi A, Pfeifer A, Ahmad M, Glauner M, Zhou XH, Ny L, Andersson KE, Kehrel B, Offermanns S, Fässler R. The vasodilator-stimulated phosphoprotein (VASP) is involved in cGMP- and cAMP-mediated inhibition of agonist-induced platelet aggregation, but is dispensable for smooth muscle function. EMBO J 18: 37–48, 1999. doi: 10.1093/emboj/18.1.37. - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

