Reduced density and altered regulation of rat atrial L-type Ca2+ current in heart failure
- PMID: 27923791
- PMCID: PMC5402008
- DOI: 10.1152/ajpheart.00528.2016
Reduced density and altered regulation of rat atrial L-type Ca2+ current in heart failure
Abstract
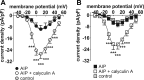
Constitutive regulation by PKA has recently been shown to contribute to L-type Ca2+ current (ICaL) at the ventricular t-tubule in heart failure. Conversely, reduction in constitutive regulation by PKA has been proposed to underlie the downregulation of atrial ICaL in heart failure. The hypothesis that downregulation of atrial ICaL in heart failure involves reduced channel phosphorylation was examined. Anesthetized adult male Wistar rats underwent surgical coronary artery ligation (CAL, N=10) or equivalent sham-operation (Sham, N=12). Left atrial myocytes were isolated ~18 wk postsurgery and whole cell currents recorded (holding potential=-80 mV). ICaL activated by depolarizing pulses to voltages from -40 to +50 mV were normalized to cell capacitance and current density-voltage relations plotted. CAL cell capacitances were ~1.67-fold greater than Sham (P ≤ 0.0001). Maximal ICaL conductance (Gmax ) was downregulated more than 2-fold in CAL vs. Sham myocytes (P < 0.0001). Norepinephrine (1 μmol/l) increased Gmax >50% more effectively in CAL than in Sham so that differences in ICaL density were abolished. Differences between CAL and Sham Gmax were not abolished by calyculin A (100 nmol/l), suggesting that increased protein dephosphorylation did not account for ICaL downregulation. Treatment with either H-89 (10 μmol/l) or AIP (5 μmol/l) had no effect on basal currents in Sham or CAL myocytes, indicating that, in contrast to ventricular myocytes, neither PKA nor CaMKII regulated basal ICaL Expression of the L-type α1C-subunit, protein phosphatases 1 and 2A, and inhibitor-1 proteins was unchanged. In conclusion, reduction in PKA-dependent regulation did not contribute to downregulation of atrial ICaL in heart failure.NEW & NOTEWORTHY Whole cell recording of L-type Ca2+ currents in atrial myocytes from rat hearts subjected to coronary artery ligation compared with those from sham-operated controls reveals marked reduction in current density in heart failure without change in channel subunit expression and associated with altered phosphorylation independent of protein kinase A.
Keywords: atrial remodeling; coronary artery ligation; voltage-gated Ca2+ channel.
Copyright © 2017 the American Physiological Society.
Figures




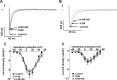

References
-
- Benjamin EJ, Chen P-S, Bild DE, Mascette AM, Albert CM, Alonso A, Calkins H, Connolly SJ, Curtis AB, Darbar D, Ellinor PT, Go AS, Goldschlager NF, Heckbert SR, Jalife J, Kerr CR, Levy D, Lloyd-Jones DM, Massie BM, Nattel S, Olgin JE, Packer DL, Po SS, Tsang TSM, Van Wagoner DR, Waldo AL, Wyse DG. Prevention of atrial fibrillation: report from a national heart, lung, and blood institute workshop. Circulation 119: 606–618, 2009. doi:10.1161/CIRCULATIONAHA.108.825380. - DOI - PMC - PubMed
-
- Boixel C, Tessier S, Pansard Y, Lang-Lazdunski L, Mercadier J-J, Hatem SN. Tyrosine kinase and protein kinase C regulate L-type Ca(2+) current cooperatively in human atrial myocytes. Am J Physiol Heart Circ Physiol 278: H670–H676, 2000. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

