An l- to d-Amino Acid Conversion in an Endosomolytic Analog of the Cell-penetrating Peptide TAT Influences Proteolytic Stability, Endocytic Uptake, and Endosomal Escape
- PMID: 27923812
- PMCID: PMC5247658
- DOI: 10.1074/jbc.M116.759837
An l- to d-Amino Acid Conversion in an Endosomolytic Analog of the Cell-penetrating Peptide TAT Influences Proteolytic Stability, Endocytic Uptake, and Endosomal Escape
Abstract
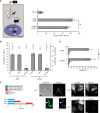
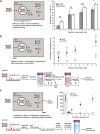
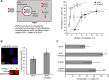
Cell-penetrating peptides (CPPs) are well established as delivery agents for otherwise cell-impermeable cargos. CPPs can also theoretically be used to modulate intracellular processes. However, their susceptibility to proteolytic degradation often limits their utility in these applications. Previous studies have explored the consequences for cellular uptake of converting the residues in CPPs from l- to d-stereochemistry, but conflicting results have been reported and specific steps en route to intracellular activity have not been explored. Here we use dimeric fluorescence TAT as a model CPP to explore the broader consequences of l- to d-stereochemical conversion. We show that inversion of chirality provides protease resistance without altering the overall mode of cellular entry, a process involving endocytic uptake followed by endosomal escape and cytosolic access. However, whereas inversion of chirality reduces endocytic uptake, the d-peptide, once in the endosome, is significantly more prone to escape than its l-counterpart. Moreover, the d-peptide is retained in the cytosol of cells for several days, whereas the l-peptide is degraded within hours. Notably, while the l-peptide is relatively innocuous to cells, the d-peptide exerts a prolonged anti-proliferative activity. Together, our results establish connections between chirality, protease resistance, cellular penetration, and intracellular activity that may be useful for the development of future delivery agents with improved properties.
Keywords: cell permeabilization; cell proliferation; cell-penetrating peptide (CPP); drug delivery system; peptide chemical synthesis; peptides.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



Similar articles
-
Efficient Delivery of Macromolecules into Human Cells by Improving the Endosomal Escape Activity of Cell-Penetrating Peptides: Lessons Learned from dfTAT and its Analogs.Biomolecules. 2018 Jul 11;8(3):50. doi: 10.3390/biom8030050. Biomolecules. 2018. PMID: 29997347 Free PMC article. Review.
-
Discovery and Mechanism of Highly Efficient Cyclic Cell-Penetrating Peptides.Biochemistry. 2016 May 10;55(18):2601-12. doi: 10.1021/acs.biochem.6b00226. Epub 2016 Apr 28. Biochemistry. 2016. PMID: 27089101 Free PMC article.
-
Plasma membrane depolarization reveals endosomal escape incapacity of cell-penetrating peptides.Eur J Pharm Biopharm. 2023 Mar;184:116-124. doi: 10.1016/j.ejpb.2023.01.019. Epub 2023 Jan 26. Eur J Pharm Biopharm. 2023. PMID: 36709921
-
Novel cell-penetrating peptides based on α-aminoxy acids.Chembiochem. 2012 Jan 2;13(1):73-9. doi: 10.1002/cbic.201100682. Epub 2011 Dec 12. Chembiochem. 2012. PMID: 22162305
-
Cell penetration: scope and limitations by the application of cell-penetrating peptides.J Pept Sci. 2014 Oct;20(10):760-84. doi: 10.1002/psc.2672. Epub 2014 Aug 11. J Pept Sci. 2014. PMID: 25112216 Review.
Cited by
-
In Vitro Profiling of the Antiviral Peptide TAT-I24.Int J Mol Sci. 2024 Sep 28;25(19):10463. doi: 10.3390/ijms251910463. Int J Mol Sci. 2024. PMID: 39408791 Free PMC article.
-
In vitro mitochondrial-targeted antioxidant peptide induces apoptosis in cancer cells.Onco Targets Ther. 2019 Sep 6;12:7297-7306. doi: 10.2147/OTT.S207640. eCollection 2019. Onco Targets Ther. 2019. PMID: 31686844 Free PMC article.
-
Mechanistic Insights into the Tools for Intracellular Protein Delivery.Chem Bio Eng. 2024 Dec 23;2(3):132-155. doi: 10.1021/cbe.4c00168. eCollection 2025 Mar 27. Chem Bio Eng. 2024. PMID: 40171130 Free PMC article. Review.
-
Cytosolic Delivery of Macromolecules in Live Human Cells Using the Combined Endosomal Escape Activities of a Small Molecule and Cell Penetrating Peptides.ACS Chem Biol. 2019 Dec 20;14(12):2641-2651. doi: 10.1021/acschembio.9b00585. Epub 2019 Oct 31. ACS Chem Biol. 2019. PMID: 31633910 Free PMC article.
-
Enhanced Live-Cell Delivery of Synthetic Proteins Assisted by Cell-Penetrating Peptides Fused to DABCYL.Angew Chem Int Ed Engl. 2021 Mar 22;60(13):7333-7343. doi: 10.1002/anie.202016208. Epub 2021 Feb 22. Angew Chem Int Ed Engl. 2021. PMID: 33615660 Free PMC article.
References
-
- Bechara C., and Sagan S. (2013) Cell-penetrating peptides: 20 years later, where do we stand? FEBS Lett. 587, 1693–1702 - PubMed
-
- Zorko M., and Langel U. (2005) Cell-penetrating peptides: mechanism and kinetics of cargo delivery. Advanced Drug Deliv. Rev. 57, 529–545 - PubMed
-
- Gupta B., Levchenko T. S., and Torchilin V. P. (2005) Intracellular delivery of large molecules and small particles by cell-penetrating proteins and peptides. Adv. Drug Deliv. Rev. 57, 637–651 - PubMed
-
- Krosl J., Austin P., Beslu N., Kroon E., Humphries R. K., and Sauvageau G. (2003) In vitro expansion of hematopoietic stem cells by recombinant TAT-HOXB4 protein. Nat. Med. 9, 1428–1432 - PubMed
-
- Morris M. C., Depollier J., Mery J., Heitz F., and Divita G. (2001) A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nat. Biotechnol. 19, 1173–1176 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

