The Chemical Basis of Thiol Addition to Nitro-conjugated Linoleic Acid, a Protective Cell-signaling Lipid
- PMID: 27923813
- PMCID: PMC5270462
- DOI: 10.1074/jbc.M116.756288
The Chemical Basis of Thiol Addition to Nitro-conjugated Linoleic Acid, a Protective Cell-signaling Lipid
Abstract
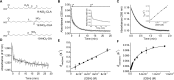
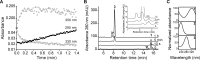
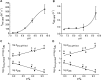
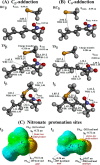
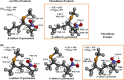
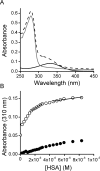
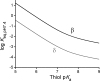
Nitroalkene fatty acids are formed in vivo and exert protective and anti-inflammatory effects via reversible Michael addition to thiol-containing proteins in key signaling pathways. Nitro-conjugated linoleic acid (NO2-CLA) is preferentially formed, constitutes the most abundant nitrated fatty acid in humans, and contains two carbons that could potentially react with thiols, modulating signaling actions and levels. In this work, we examined the reactions of NO2-CLA with low molecular weight thiols (glutathione, cysteine, homocysteine, cysteinylglycine, and β-mercaptoethanol) and human serum albumin. Reactions followed reversible biphasic kinetics, consistent with the presence of two electrophilic centers in NO2-CLA located on the β- and δ-carbons with respect to the nitro group. The differential reactivity was confirmed by computational modeling of the electronic structure. The rates (kon and koff) and equilibrium constants for both reactions were determined for different thiols. LC-UV-Visible and LC-MS analyses showed that the fast reaction corresponds to β-adduct formation (the kinetic product), while the slow reaction corresponds to the formation of the δ-adduct (the thermodynamic product). The pH dependence of the rate constants, the correlation between intrinsic reactivity and thiol pKa, and the absence of deuterium solvent kinetic isotope effects suggested stepwise mechanisms with thiolate attack on NO2-CLA as rate-controlling step. Computational modeling supported the mechanism and revealed additional features of the transition states, anionic intermediates, and final neutral products. Importantly, the detection of cysteine-δ-adducts in human urine provided evidence for the biological relevance of this reaction. Finally, human serum albumin was found to bind NO2-CLA both non-covalently and to form covalent adducts at Cys-34, suggesting potential modes for systemic distribution. These results provide new insights into the chemical basis of NO2-CLA signaling actions.
Keywords: Michael addition; albumin; conjugated nitrolinoleic acid; elimination; fatty acid; kinetics; nitro fatty acid; nitroalkene fatty acid; sulfhydryl; thiol.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



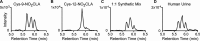
References
-
- Delmastro-Greenwood M., Hughan K. S., Vitturi D. A., Salvatore S. R., Grimes G., Potti G., Shiva S., Schopfer F. J., Gladwin M. T., Freeman B. A., and Gelhaus Wendell S. (2015) Nitrite and nitrate-dependent generation of anti-inflammatory fatty acid nitroalkenes. Free Radic. Biol. Med. 89, 333–341 - PMC - PubMed
-
- Vitturi D. A., Chen C. S., Woodcock S. R., Salvatore S. R., Bonacci G., Koenitzer J. R., Stewart N. A., Wakabayashi N., Kensler T. W., Freeman B. A., and Schopfer F. J. (2013) Modulation of nitro-fatty acid signaling: prostaglandin reductase-1 is a nitroalkene reductase. J. Biol. Chem. 288, 25626–25637 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

