Deficiency of ATP6V1H Causes Bone Loss by Inhibiting Bone Resorption and Bone Formation through the TGF-β1 Pathway
- PMID: 27924156
- PMCID: PMC5135442
- DOI: 10.7150/thno.17140
Deficiency of ATP6V1H Causes Bone Loss by Inhibiting Bone Resorption and Bone Formation through the TGF-β1 Pathway
Abstract
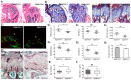
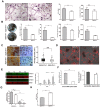
Vacuolar-type H +-ATPase (V-ATPase) is a highly conserved, ancient enzyme that couples the energy of ATP hydrolysis to proton transport across vesicular and plasma membranes of eukaryotic cells. Previously reported mutations of various V-ATPase subunits are associated with increased bone density. We now show that haploinsufficiency for the H subunit of the V1 domain (ATP6V1H) is associated with osteoporosis in humans and mice. A genome-wide SNP array analysis of 1625 Han Chinese found that 4 of 15 tag SNPs (26.7%) within ATP6V1H were significantly associated with low spine bone mineral density. Atp6v1h+/- knockout mice generated by the CRISPR/Cas9 technique had decreased bone remodeling and a net bone matrix loss. Atp6v1h+/- osteoclasts showed impaired bone formation and increased bone resorption. The increased intracellular pH of Atp6v1h+/- osteoclasts downregulated TGF-β1 activation, thereby reducing induction of osteoblast formation but the bone mineralization was not altered. However, bone formation was reduced more than bone resorption. Our data provide evidence that partial loss of ATP6V1H function results in osteoporosis/osteopenia. We propose that defective osteoclast formation triggers impaired bone formation by altering bone remodeling. In the future, ATP6V1H might, therefore, serve as a target for the therapy of osteoporosis.
Keywords: ATP6V1H; CRISPR/Cas9; OPG.; RANKL; TGF-β1; V-ATPase; osteoclasts; osteoporosis; pH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Yao G, Feng H, Cai Y, Qi W, Kong K. Characterization of vacuolar-ATPase and selective inhibition of vacuolar-H(+)-ATPase in osteoclasts. Biochem Biophys Res Commun. 2007;357:821–7. - PubMed
-
- Frattini A, Orchard PJ, Sobacchi C, Giliani S, Abinun M, Mattsson JP. et al. Defects in TCIRG1 subunit of the vacuolar proton pump are responsible for a subset of human autosomal recessive osteopetrosis. Nat Genet. 2000;25:343–6. - PubMed
-
- Susani L, Pangrazio A, Sobacchi C, Taranta A, Mortier G, Savarirayan R. et al. TCIRG1-dependent recessive osteopetrosis: mutation analysis, functional identification of the splicing defects, and in vitro rescue by U1 snRNA. Hum Mutat. 2004;24:225–35. - PubMed
-
- Scimeca JC, Quincey D, Parrinello H, Romatet D, Grosgeorge J, Gaudray P. et al. Novel mutations in the TCIRG1 gene encoding the a3 subunit of the vacuolar proton pump in patients affected by infantile malignant osteopetrosis. Hum Mutat. 2003;21:151–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

