Caffeine combined with sedative/anesthetic drugs triggers widespread neuroapoptosis in a mouse model of prematurity
- PMID: 27924651
- PMCID: PMC5462883
- DOI: 10.1080/14767058.2016.1261400
Caffeine combined with sedative/anesthetic drugs triggers widespread neuroapoptosis in a mouse model of prematurity
Abstract
Objectives: Caffeine (CAF) and sedative/anesthetic drugs (SADs) are often coadministered to premature infants in the neonatal intensive care unit (NICU). While SAD neurotoxicity in the developing brain is well established, it is not fully clear whether CAF interacts with SADs and whether this interaction is detrimental. Using a mouse model of prematurity, we hypothesized that CAF would increase apoptotic neurotoxicity when coadministered with SADs.
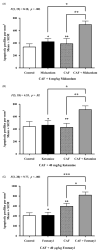
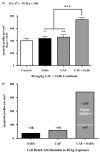
Methods: Postnatal day 3 mice were treated with vehicle or 80 mg/kg CAF prior to challenge with 6 mg/kg midazolam, 40 mg/kg ketamine, or 40 μg/kg fentanyl. Six hours later, pups were sacrificed for activated caspase 3 (AC3) immunohistochemistry, and number of AC3 positive cells per mm3 throughout neocortex, hippocampus, caudate, thalamus, and colliculi was analyzed.
Results: CAF caused a statistically significant increase in AC3 positive cells when coadministered with midazolam (p = 0.002), ketamine (p = 0.014), or fentanyl (p < 0.001). Our composite dataset suggests that the addition of CAF to these SADs has a supra-additive effect, causing more neurotoxicity than expected.
Conclusions: CAF may augment the neurotoxic action of SADs indicated for neonatal sedation/anesthesia in the NICU by triggering widespread apoptosis in the developing brains of premature infants.
Keywords: Caffeine; apoptosis; fentanyl; ketamine; midazolam; premature infant.
Conflict of interest statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Figures

References
-
- Filan PM, Hunt RW, Anderson PJ, et al. Neurologic outcomes in very preterm infants undergoing surgery. J Pediatr. 2012;160:409–14. - PubMed
-
- Henderson-Smart DJ, Steer P. Methylxanthine treatment for apnea in preterm infants. Cochrane Database Syst Rev. 2001:CD000140. - PubMed
-
- Schmidt B, Roberts RS, Davis P, et al. Caffeine for Apnea of Prematurity Trial G. Caffeine therapy for apnea of prematurity. N Engl J Med. 2006;354:2112–21. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
