Predictive biomarkers for checkpoint inhibitor-based immunotherapy
- PMID: 27924752
- PMCID: PMC5702534
- DOI: 10.1016/S1470-2045(16)30406-5
Predictive biomarkers for checkpoint inhibitor-based immunotherapy
Abstract
The clinical development of checkpoint inhibitor-based immunotherapy has ushered in an exciting era of anticancer therapy. Durable responses can be seen in patients with melanoma and other malignancies. Although monotherapy with PD-1 or PD-L1 agents are typically well tolerated, the risk of immune-related adverse events increases with combination regimens. The development of predictive biomarkers is needed to optimise patient benefit, minimise risk of toxicities, and guide combination approaches. The greatest focus has been on tumour-cell PD-L1 expression. Although PD-L1 positivity enriches for populations with clinical benefit, PD-L1 testing alone is insufficient for patient selection in most malignancies. In this Review, we discuss the status of PD-L1 testing and explore emerging data on new biomarker strategies with tumour-infiltrating lymphocytes, mutational burden, immune gene signatures, and multiplex immunohistochemistry. Future development of an effective predictive biomarker for checkpoint inhibitor-based immunotherapy will integrate multiple approaches for optimal characterisation of the immune tumour microenvironment.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
GTG reports personal fees from Novartis and Merck. LMW reports personal fees from Merck, Genentech, Jounce Pharmaceuticals, Celldex Pharmaceuticals, and Merrimack Pharmaceuticals. MBA reports personal fees from Bristol-Myers Squibb, Merck, Genentech/Roche, Novartis, Pfizer, and Astra Zeneca.
Figures
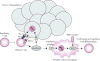
References
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. - PubMed
-
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. - PubMed
-
- Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

