Passive therapy with humanized anti-staphylococcal enterotoxin B antibodies attenuates systemic inflammatory response and protects from lethal pneumonia caused by staphylococcal enterotoxin B-producing Staphylococcus aureus
- PMID: 27925510
- PMCID: PMC5711449
- DOI: 10.1080/21505594.2016.1267894
Passive therapy with humanized anti-staphylococcal enterotoxin B antibodies attenuates systemic inflammatory response and protects from lethal pneumonia caused by staphylococcal enterotoxin B-producing Staphylococcus aureus
Abstract
Drugs such as linezolid that inhibit bacterial protein synthesis may be beneficial in treating infections caused by toxigenic Staphylococcus aureus. As protein synthesis inhibitors have no effect on preformed toxins, neutralization of pathogenic exotoxins with anti-toxin antibodies may be beneficial in conjunction with antibacterial therapy. Herein, we evaluated the efficacy of human-mouse chimeric high-affinity neutralizing anti-staphylococcal enterotoxin B (SEB) antibodies in the treatment of experimental pneumonia caused by SEB-producing S. aureus. Since HLA class II transgenic mice mount a stronger systemic immune response following challenge with SEB and are more susceptible to SEB-induced lethal toxic shock than conventional mice strains, HLA-DR3 transgenic mice were used. Lethal pneumonia caused by SEB-producing S. aureus in HLA-DR3 transgenic mice was characterized by robust T cell activation and elevated systemic levels of several pro-inflammatory cytokines and chemokines. Prophylactic administration of a single dose of linezolid 30 min prior to the onset of infection attenuated the systemic inflammatory response and protected from mortality whereas linezolid administered 60 min after the onset of infection failed to confer significant protection. Human-mouse chimeric high-affinity neutralizing anti-SEB antibodies alone, but not polyclonal human IgG, mitigated this response and protected from death when administered immediately after initiation of infection. Further, anti-SEB antibodies as well as intact polyclonal human IgG, but not its Fab or Fc fragments, protected from lethal pneumonia when followed with linezolid therapy 60 min later. In conclusion, neutralization of superantigens with high-affinity antibodies may have beneficial effects in pneumonia.
Keywords: HLA class II transgenic mice; T lymphocytes; pneumonia; staphylococcus aureus; superantigen.
Figures

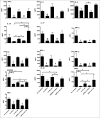
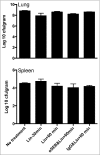
Comment in
-
Staphylococcus aureus toxin antibodies: Good companions of antibiotics and vaccines.Virulence. 2017 Oct 3;8(7):1037-1042. doi: 10.1080/21505594.2017.1295205. Epub 2017 Mar 7. Virulence. 2017. PMID: 28267417 Free PMC article. No abstract available.
References
-
- Lowy FD. Staphylococcus aureus Infections. N Engl J Med 1998; 339:520-32; PMID:9709046; http://dx.doi.org/10.1056/NEJM199808203390806 - DOI - PubMed
-
- DeLeo FR, Otto M, Kreiswirth BN, Chambers HF. Community-associated meticillin-resistant Staphylococcus aureus. Lancet 2010; 375:1557-68; PMID:20206987; http://dx.doi.org/10.1016/S0140-6736(09)61999-1 - DOI - PMC - PubMed
-
- Otter JA, French GL. Molecular epidemiology of community-associated meticillin-resistant Staphylococcus aureus in Europe. Lancet Infect Dis 2010; 10:227-39; PMID:20334846; http://dx.doi.org/10.1016/S1473-3099(10)70053-0 - DOI - PubMed
-
- Bassetti M, Nicco E, Mikulska M. Why is community-associated MRSA spreading across the world and how will it change clinical practice? Int J Antimicrob Agents 2009; 34:S15-S9; PMID:19560669; http://dx.doi.org/10.1016/S0924-8579(09)70544-8 - DOI - PubMed
-
- Schlievert PM. Staphylococcal toxic shock syndrome: still a problem. Med J Aust 2005; 182:651-2; PMID:16116687 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
