Cyclic Immunofluorescence (CycIF), A Highly Multiplexed Method for Single-cell Imaging
- PMID: 27925668
- PMCID: PMC5233430
- DOI: 10.1002/cpch.14
Cyclic Immunofluorescence (CycIF), A Highly Multiplexed Method for Single-cell Imaging
Abstract
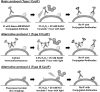
Cyclic Immunofluorescence (CycIF) is a public-domain method for performing highly multiplexed immunofluorescence imaging using a conventional epifluorescence microscope. It uses simple reagents and existing antibodies to construct images with up to 30 channels by sequential 4- to 6-channel imaging followed by fluorophore inactivation. Three variant methods are described, the most generally useful of which involves staining fixed cells with antibodies directly conjugated to Alexa Fluor dyes and imaging in four colors, inactivating fluorophores using a mild base in the presence of hydrogen peroxide and light, and then performing another round of staining and imaging. Cell morphology is preserved through multiple rounds of CycIF, and signal-to-noise ratios appear to increase. Unlike antibody-stripping methods, CycIF is gentle and optimized for monolayers of cultured cells. A second protocol involves indirect immunofluorescence and a third enables chemical inactivation of genetically encoded fluorescent proteins, allowing multiplex immunofluorescence to be combined with live-cell analysis of cells expressing fluorescent reporter proteins. © 2016 by John Wiley & Sons, Inc.
Keywords: CycIF; high-content imaging; immunofluorescence; multiplexing; systems biology.
Copyright © 2016 John Wiley & Sons, Inc.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

