A two-dose heterologous prime-boost vaccine regimen eliciting sustained immune responses to Ebola Zaire could support a preventive strategy for future outbreaks
- PMID: 27925844
- PMCID: PMC5328205
- DOI: 10.1080/21645515.2017.1264755
A two-dose heterologous prime-boost vaccine regimen eliciting sustained immune responses to Ebola Zaire could support a preventive strategy for future outbreaks
Abstract
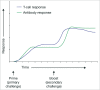
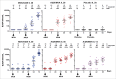
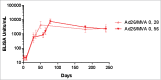
The consequences of the 2013-16 Ebola Zaire virus disease epidemic in West Africa were grave. The economies, healthcare systems and communities of Guinea, Sierra Leone and Liberia were devastated by over 18 months of active Ebola virus transmission, followed by sporadic resurgences potentially related to sexual transmission by survivors with viral persistence in body fluids following recovery. The need to develop and implement strategies to prevent and mitigate future outbreaks is now beyond dispute. The potential for unpredictable outbreaks of indeterminate duration, and control challenges posed by the possibility of sporadic re-emergence, mean that implementation of an effective vaccination program for outbreak containment necessitates a vaccine providing durable immunity. Heterologous prime-boost vaccine regimens deliver the same or similar antigens through different vaccine types, the first to prime and the second to boost the immune system. Ad26.ZEBOV/MVA-BN-Filo is an investigational Ebola Zaire vaccine regimen that uses this heterologous prime-boost approach. Preliminary Phase 1 data suggest that Ad26.ZEBOV/MVA-BN-Filo confers durable immunity for at least 240 d and is well-tolerated with a good safety profile. This regimen may therefore be suitable for prophylactic use in a regional or targeted population vaccination strategy, and could potentially aid prevention and control of future Ebola outbreaks.
Keywords: Ad26.ZEBOV/MVA-BN-Filo; Ebola virus; heterologous prime-boost; population vaccination strategy; sustained immunity; viral persistence.
Figures

References
-
- Pringle CR. Virus taxonomy—San Diego 1998. Arch Virol 1998; 143:1449-60; PMID:9742051; http://dx.doi.org/10.1007/s007050050389 - DOI - PubMed
-
- Brainard J, Pond K, Hooper L, Edmunds K, Hunter P. Presence and persistence of ebola or Marburg virus in patients and survivors: a rapid systematic review. PLoS Negl Trop Dis 2016; 10:e0004475; PMID:26927697; http://dx.doi.org/10.1371/journal.pntd.0004475 - DOI - PMC - PubMed
-
- Centres for Disease Control and Prevention Ebola (Ebola virus disease) About Ebola Virus Disease. 2016a. http://www.cdc.gov/vhf/ebola/about.html. Last accessed: 26August2016.
-
- Centres for Disease Control and Prevention Ebola (Ebola virus disease) Case counts. 2016b. https://www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/. Last accessed: 26August2016.
-
- Chughtai AA, Barnes M, Macintyre CR. Persistence of Ebola virus in various body fluids during convalescence: evidence and implications for disease control and transmission. Epidemiol Infect 2016; 144:1652-60; PMID:26808232; http://dx.doi.org/10.1017/S0950268816000054 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
