Clonal haemopoiesis and therapy-related myeloid malignancies in elderly patients: a proof-of-concept, case-control study
- PMID: 27927582
- PMCID: PMC7771361
- DOI: 10.1016/S1470-2045(16)30627-1
Clonal haemopoiesis and therapy-related myeloid malignancies in elderly patients: a proof-of-concept, case-control study
Abstract
Background: Clonal haemopoiesis of indeterminate potential (CHIP) is an age-associated genetic event linked to increased risk of primary haematological malignancies and increased all-cause mortality, but the prevalence of CHIP in patients who develop therapy-related myeloid neoplasms is unknown. We did this study to investigate whether chemotherapy-treated patients with cancer who have CHIP are at increased risk of developing therapy-related myeloid neoplasms.
Methods: We did a nested, case-control, proof-of-concept study to compare the prevalence of CHIP between patients with cancer who later developed therapy-related myeloid neoplasms (cases) and patients who did not develop these neoplasms (controls). We identified cases from our internal biorepository of 123 357 patients who consented to participate in the Total Cancer Care biobanking protocol at Moffitt Cancer Center (Tampa, FL, USA) between Jan 1, 2006, and June 1, 2016. We included all individuals who were diagnosed with a primary malignancy, were treated with chemotherapy, subsequently developed a therapy-related myeloid neoplasm, and were 70 years or older at either diagnosis. For inclusion in this study, individuals must have had a peripheral blood or mononuclear cell sample collected before the diagnosis of therapy-related myeloid neoplasm. Controls were individuals who were diagnosed with a primary malignancy at age 70 years or older and were treated with chemotherapy but did not develop therapy-related myeloid neoplasms. Controls were matched to cases in at least a 4:1 ratio on the basis of sex, primary tumour type, age at diagnosis, smoking status, chemotherapy drug class, and duration of follow-up. We used sequential targeted and whole-exome sequencing and described clonal evolution in cases for whom paired CHIP and therapy-related myeloid neoplasm samples were available. The primary endpoint of this study was the development of therapy-related myeloid neoplasm and the primary exposure was CHIP.
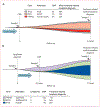
Findings: We identified 13 cases and 56 case-matched controls. The prevalence of CHIP in all patients (23 [33%] of 69 patients) was higher than has previously been reported in elderly individuals without cancer (about 10%). Cases had a significantly higher prevalence of CHIP than did matched controls (eight [62%] of 13 cases vs 15 [27%] of 56 controls, p=0·024; odds ratio 5·75, 95% CI 1·52-25·09, p=0·013). The most commonly mutated genes in cases with CHIP were TET2 (three [38%] of eight patients) and TP53(three [38%] of eight patients), whereas controls most often had TET2 mutations (six [40%] of 15 patients). In most (four [67%] of six patients) cases for whom paired CHIP and therapy-related myeloid neoplasm samples were available, the mean allele frequency of CHIP mutations had expanded by the time of the therapy-related myeloid neoplasm diagnosis. However, a subset of paired samples (two [33%] of six patients) had CHIP mutations that decreased in allele frequency, giving way to expansion of a distinct mutant clone.
Interpretation: Patients with cancer who have CHIP are at increased risk of developing therapy-related myeloid neoplasms. The distribution of CHIP-related gene mutations differs between individuals with therapy-related myeloid neoplasm and those without, suggesting that mutation-specific differences might exist in therapy-related myeloid neoplasm risk.
Funding: Moffitt Cancer Center.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures

Comment in
-
Predicting therapy-related myeloid neoplasms-and preventing them?Lancet Oncol. 2017 Jan;18(1):11-13. doi: 10.1016/S1470-2045(16)30622-2. Epub 2016 Dec 4. Lancet Oncol. 2017. PMID: 27927581 No abstract available.
References
-
- Malmgren JA, Calip GS, Pyott SM, Atwood MK, Kaplan HG. Therapy-related myelodysplastic syndrome following primary breast cancer. Leuk Res 2016; 47: 178–84. - PubMed
-
- Ben-Yehuda D, Krichevsky S, Caspi O, et al. Microsatellite instability and p53 mutations in therapy-related leukemia suggest mutator phenotype. Blood 1996; 88: 4296–303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

