Lysophosphatidic acid signaling through its receptor initiates profibrotic epithelial cell fibroblast communication mediated by epithelial cell derived connective tissue growth factor
- PMID: 27927603
- PMCID: PMC5313343
- DOI: 10.1016/j.kint.2016.09.030
Lysophosphatidic acid signaling through its receptor initiates profibrotic epithelial cell fibroblast communication mediated by epithelial cell derived connective tissue growth factor
Abstract
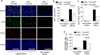
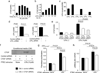
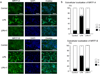
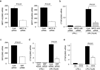
The expansion of the fibroblast pool is a critical step in organ fibrosis, but the mechanisms driving expansion remain to be fully clarified. We previously showed that lysophosphatidic acid (LPA) signaling through its receptor LPA1 expressed on fibroblasts directly induces the recruitment of these cells. Here we tested whether LPA-LPA1 signaling drives fibroblast proliferation and activation during the development of renal fibrosis. LPA1-deficient (LPA1-/-) or -sufficient (LPA1+/+) mice were crossed to mice with green fluorescent protein expression (GFP) driven by the type I procollagen promoter (Col-GFP) to identify fibroblasts. Unilateral ureteral obstruction-induced increases in renal collagen were significantly, though not completely, attenuated in LPA1-/-Col-GFP mice, as were the accumulations of both fibroblasts and myofibroblasts. Connective tissue growth factor was detected mainly in tubular epithelial cells, and its levels were suppressed in LPA1-/-Col-GFP mice. LPA-LPA1 signaling directly induced connective tissue growth factor expression in primary proximal tubular epithelial cells, through a myocardin-related transcription factor-serum response factor pathway. Proximal tubular epithelial cell-derived connective tissue growth factor mediated renal fibroblast proliferation and myofibroblast differentiation. Administration of an inhibitor of myocardin-related transcription factor/serum response factor suppressed obstruction-induced renal fibrosis. Thus, targeting LPA-LPA1 signaling and/or myocardin-related transcription factor/serum response factor-induced transcription could be promising therapeutic strategies for renal fibrosis.
Keywords: CTGF; LPA(1); fibroblast; fibrosis.
Copyright © 2016 International Society of Nephrology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no conflicting financial interests.
Figures







References
-
- Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–151. - PubMed
-
- Scotton CJ, Chambers RC. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest. 2007;132:1311–1321. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

