Prenatal myonuclei play a crucial role in skeletal muscle hypertrophy in rodents
- PMID: 27927611
- PMCID: PMC5401943
- DOI: 10.1152/ajpcell.00151.2016
Prenatal myonuclei play a crucial role in skeletal muscle hypertrophy in rodents
Abstract
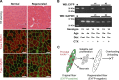
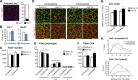
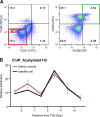
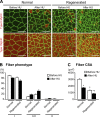
Multinucleated muscle fibers are formed by the fusion of myogenic progenitor cells during embryonic and fetal myogenesis. However, the role of prenatally incorporated myonuclei in the skeletal muscle fibers of adult animals is poorly understood. We demonstrated, using muscle-specific reporter mice, that the prenatal myonuclei remained in the adult soleus muscle, although cardiotoxin injection caused the loss of prenatal myonuclei. Overloading by the tendon transection of synergists failed to induce compensatory hypertrophy in regenerated soleus muscle fibers of adult rats, whereas unloading by tail suspension normally induced the fiber atrophy. Loss of hypertrophying function correlated with the lowered histone acetylation at the transcription start site of Igf1r gene, which was one of the genes that did not respond to the overloading. These parameters were improved by the transplantation of cells harvested from the juvenile soleus muscles of neonatal rats in association with enhanced histone acetylation of Igf1r gene. These results indicated that the presence of prenatal myonuclei was closely related to the status of histone acetylation, which could regulate the responsiveness of muscle fibers to physiological stimuli.
Keywords: histone modification; injury; myonucleus; regeneration; skeletal muscle fiber.
Copyright © 2017 the American Physiological Society.
Figures

Similar articles
-
Myonucleus-related properties in soleus muscle fibers of mdx mice.Cells Tissues Organs. 2010;191(3):248-59. doi: 10.1159/000240245. Epub 2009 Sep 18. Cells Tissues Organs. 2010. PMID: 19776548
-
IGF-II is up-regulated and myofibres are hypertrophied in regenerating soleus of mice lacking FGF6.Exp Cell Res. 2004 Jul 1;297(1):27-38. doi: 10.1016/j.yexcr.2004.02.021. Exp Cell Res. 2004. PMID: 15194422
-
Exclusive vital labeling of myonuclei for studying myonuclear arrangement in mouse skeletal muscle tissue.Skelet Muscle. 2020 May 7;10(1):15. doi: 10.1186/s13395-020-00233-6. Skelet Muscle. 2020. PMID: 32381068 Free PMC article.
-
Muscular dystrophy: centronucleation may reflect a compensatory activation of defective myonuclei.J Biomed Sci. 1998;5(1):54-61. doi: 10.1007/BF02253356. J Biomed Sci. 1998. PMID: 9570514 Review.
-
Myonuclear domains in muscle adaptation and disease.Muscle Nerve. 1999 Oct;22(10):1350-60. doi: 10.1002/(sici)1097-4598(199910)22:10<1350::aid-mus3>3.0.co;2-8. Muscle Nerve. 1999. PMID: 10487900 Review.
Cited by
-
At the Nexus Between Epigenetics and Senescence: The Effects of Senolytic (BI01) Administration on DNA Methylation Clock Age and the Methylome in Aged and Regenerated Skeletal Muscle.Aging Cell. 2025 Jul;24(7):e70068. doi: 10.1111/acel.70068. Epub 2025 Apr 21. Aging Cell. 2025. PMID: 40259517 Free PMC article.
-
Early satellite cell communication creates a permissive environment for long-term muscle growth.iScience. 2021 Mar 29;24(4):102372. doi: 10.1016/j.isci.2021.102372. eCollection 2021 Apr 23. iScience. 2021. PMID: 33948557 Free PMC article.
-
Starring or Supporting Role? Satellite Cells and Skeletal Muscle Fiber Size Regulation.Physiology (Bethesda). 2018 Jan 1;33(1):26-38. doi: 10.1152/physiol.00019.2017. Physiology (Bethesda). 2018. PMID: 29212890 Free PMC article. Review.
References
-
- Allen DL, Linderman JK, Roy RR, Bigbee AJ, Grindeland RE, Mukku V, Edgerton VR. Apoptosis: a mechanism contributing to remodeling of skeletal muscle in response to hindlimb unweighting. Am J Physiol Cell Physiol 273: C579–C587, 1997. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

