Surrogate End Points for Overall Survival in Loco-Regionally Advanced Nasopharyngeal Carcinoma: An Individual Patient Data Meta-analysis
- PMID: 27927756
- PMCID: PMC6059121
- DOI: 10.1093/jnci/djw239
Surrogate End Points for Overall Survival in Loco-Regionally Advanced Nasopharyngeal Carcinoma: An Individual Patient Data Meta-analysis
Abstract
Background: Our objective was to evaluate progression-free survival (PFS) and distant metastasis-free survival (DMFS) as surrogate end points for overall survival (OS) in randomized trials of chemotherapy in loco-regionally advanced nasopharyngeal carcinomas (NPCs).
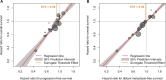
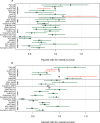
Methods: Individual patient data were obtained from 19 trials of the updated Meta-Analysis of Chemotherapy in Nasopharyngeal Carcinoma (MAC-NPC) plus one additional trial (total = 5144 patients). Surrogacy was evaluated at the individual level using a rank correlation coefficient ρ and at the trial level using a correlation coefficient R2 between treatment effects on the surrogate end point and OS. A sensitivity analysis was performed with two-year PFS/DMFS and five-year OS.
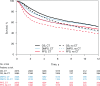
Results: PFS was strongly correlated with OS at the individual level (ρ = 0.93, 95% confidence interval [CI] = 0.93 to 0.94) and at the trial level (R2 = 0.95, 95% CI = 0.47 to 1.00). For DMFS, too, the individual-level correlation with OS was strong (ρ = 0.98, 95% CI = 0.98 to 0.98); at trial level, the correlation was high but the regression adjusted for measurement error could not be computed (unadjusted R2 = 0.96, 95% CI = 0.94 to 0.99). In the sensitivity analysis, two-year PFS was highly correlated with five-year OS at the individual level (ρ = 0.89, 95% CI = 0.88 to 0.90) and at the trial level (R2 = 0.85, 95% CI = 0.46 to 1.00); two-year DMFS was highly correlated with five-year OS at the individual level (ρ = 0.95, 95% CI = 0.94 to 0.95) and at the trial level (R2 = 0.78, 95% CI = 0.33 to 1.00).
Conclusions: PFS and DMFS are valid surrogate end points for OS to assess treatment effect of chemotherapy in loco-regionally advanced NPC, while PFS can be measured earlier.
© The Author 2016. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Accessed August 11, 2016.
-
- Blanchard P, Lee A, Marguet S, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: An update of the MAC-NPC meta-analysis. Lancet Oncol. 2015;166:645–655. - PubMed
-
- Michiels S, Le Maître A, Buyse M, et al. Surrogate endpoints for overall survival in locally advanced head and neck cancer: Meta-analyses of individual patient data. Lancet Oncol. 2009;104:341–350. - PubMed
-
- Chua MLK, Wee JTS, Hui EP, Chan ATC.. Nasopharyngeal carcinoma. Lancet. 2016;38710022:1012–1024. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

