Sleep Neurophysiological Dynamics Through the Lens of Multitaper Spectral Analysis
- PMID: 27927806
- PMCID: PMC5343535
- DOI: 10.1152/physiol.00062.2015
Sleep Neurophysiological Dynamics Through the Lens of Multitaper Spectral Analysis
Erratum in
-
Corrigendum for Prerau MJ, et al., Volume 32, 2017, p. 60-92.Physiology (Bethesda). 2023 Sep 1;38(5):0. doi: 10.1152/physiol.00062.2015_COR. Physiology (Bethesda). 2023. PMID: 37466325 Free PMC article. No abstract available.
Abstract
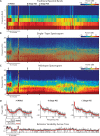
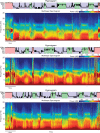
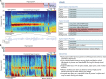
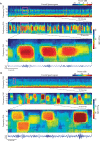
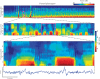
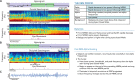
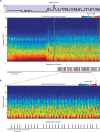
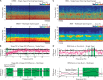
During sleep, cortical and subcortical structures within the brain engage in highly structured oscillatory dynamics that can be observed in the electroencephalogram (EEG). The ability to accurately describe changes in sleep state from these oscillations has thus been a major goal of sleep medicine. While numerous studies over the past 50 years have shown sleep to be a continuous, multifocal, dynamic process, long-standing clinical practice categorizes sleep EEG into discrete stages through visual inspection of 30-s epochs. By representing sleep as a coarsely discretized progression of stages, vital neurophysiological information on the dynamic interplay between sleep and arousal is lost. However, by using principled time-frequency spectral analysis methods, the rich dynamics of the sleep EEG are immediately visible-elegantly depicted and quantified at time scales ranging from a full night down to individual microevents. In this paper, we review the neurophysiology of sleep through this lens of dynamic spectral analysis. We begin by reviewing spectral estimation techniques traditionally used in sleep EEG analysis and introduce multitaper spectral analysis, a method that makes EEG spectral estimates clearer and more accurate than traditional approaches. Through the lens of the multitaper spectrogram, we review the oscillations and mechanisms underlying the traditional sleep stages. In doing so, we will demonstrate how multitaper spectral analysis makes the oscillatory structure of traditional sleep states instantaneously visible, closely paralleling the traditional hypnogram, but with a richness of information that suggests novel insights into the neural mechanisms of sleep, as well as novel clinical and research applications.
©2017 Int. Union Physiol. Sci./Am. Physiol. Soc.
Conflict of interest statement
M.T.B. receives funding from the Department of Neurology, Massachusetts General Hospital, the Center for Integration of Medicine and Innovative Technology, the Milton Family Foundation, and the MGH-MIT Grand Challenge. M.T.B. has a pending patent on a sleep wearable device, received travel funds from Servier, served on the advisory board of Foramis, received research funding from MC10, Inc. and Insomnisolv, Inc., has consulting agreements with McKesson and with International Flavors and Fragrances, and has provided expert testimony in sleep medicine.
M.J.P. and P.L.P. have patents pending on the monitoring of sleep and anesthesia. M.T.B. has a patent pending for a sleep monitoring device.
Figures





References
-
- Adrian E, Matthews BH. The Berger rhythm: potential changes from the occipital lobes in man. Brain 57: 355–385, 1934. - PubMed
-
- Adrian ED, Yamagiwa K. The origin of the Berger rhythm. Brain 58: 323–351, 1935.
-
- Aserinsky E, Kleitman N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science 118: 273–274, 1953. - PubMed
-
- Astori S, Wimmer RD, Luthi A. Manipulating sleep spindles: expanding views on sleep, memory, and disease. Trends Neurosci 36: 738–748, 2013. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

