Zika Virus Testing Considerations: Lessons Learned from the First 80 Real-Time Reverse Transcription-PCR-Positive Cases Diagnosed in New York State
- PMID: 27927917
- PMCID: PMC5277524
- DOI: 10.1128/JCM.01232-16
Zika Virus Testing Considerations: Lessons Learned from the First 80 Real-Time Reverse Transcription-PCR-Positive Cases Diagnosed in New York State
Abstract
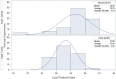
The performance and interpretation of laboratory tests for Zika virus (ZKV) continue to be evaluated. Serology is cross-reactive, laborious, and frequently difficult to interpret, and serum was initially solely recommended for molecular diagnosis. ZKV testing was initiated in January 2016 in New York State for symptomatic patients, pregnant women, their infants, and patients with Guillain-Barré syndrome who had traveled to areas with ZKV transmission. Subsequently, eligibility was expanded to pregnant women with sexual partners with similar travel histories. Serum and urine collected within 4 weeks of symptom onset or within 6 weeks of travel were tested with real-time reverse transcription-PCR (RT-PCR) assays targeting the ZKV envelope and NS2B genes. In this review of lessons learned from the first 80 positive cases in NYS, ZKV RNA was detected in urine only in 50 patients, in serum only in 19 patients, and in both samples concurrently in 11 patients, with average viral loads in urine a log higher than those in serum. Among 93 positive samples from the 80 patients, 41 were positive on both gene assays, 52 were positive on the envelope only, and none were positive on the NS2B only. Of the 80 infected patients, test results for 74 (93%) would have defined their infection status as not detected or equivocal if the requirement for positive results from two assay targets (two-target-positive requirement) in the initial federal guidance to public health laboratories was enforced, if urine was not tested, or if the extended eligibility time for molecular testing was not implemented. These changes facilitated more extensive molecular diagnosis of ZKV, reducing reliance on time-consuming and potentially inconclusive serology.
Keywords: diagnostics; emerging pathogens; molecular methods; specimen selection; viral diagnosis.
Copyright © 2017 American Society for Microbiology.
Figures



References
-
- Cao-Lormeau VM, Blake A, Mons S, Lastere S, Roche C, Vanhomwegen J, Dub T, Baudouin L, Teissier A, Larre P, Vial AL, Decam C, Choumet V, Halstead SK, Willison HJ, Musset L, Manuguerra JC, Despres P, Fournier E, Mallet HP, Musso D, Fontanet A, Neil J, Ghawche F. 2016. Guillain-Barre syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet 387:1531–1539. doi:10.1016/S0140-6736(16)00562-6. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

