Vectored Intracerebral Immunization with the Anti-Tau Monoclonal Antibody PHF1 Markedly Reduces Tau Pathology in Mutant Tau Transgenic Mice
- PMID: 27927959
- PMCID: PMC6601971
- DOI: 10.1523/JNEUROSCI.2016-16.2016
Vectored Intracerebral Immunization with the Anti-Tau Monoclonal Antibody PHF1 Markedly Reduces Tau Pathology in Mutant Tau Transgenic Mice
Erratum in
-
Correction: Liu et al., "Vectored Intracerebral Immunization with the Anti-Tau Monoclonal Antibody PHF1 Markedly Reduces Tau Pathology in Mutant Tau Transgenic Mice".J Neurosci. 2017 Mar 29;37(13):3734. doi: 10.1523/JNEUROSCI.0488-17.2017. J Neurosci. 2017. PMID: 28356396 Free PMC article. No abstract available.
Abstract
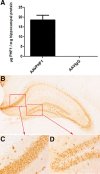
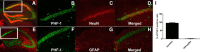
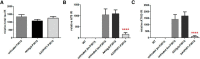
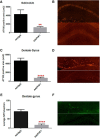
Passive immunization with anti-tau monoclonal antibodies has been shown by several laboratories to reduce age-dependent tau pathology and neurodegeneration in mutant tau transgenic mice. These studies have used repeated high weekly doses of various tau antibodies administered systemically for several months and have reported reduced tau pathology of ∼40-50% in various brain regions. Here we show that direct intrahippocampal administration of the adeno-associated virus (AAV)-vectored anti-phospho-tau antibody PHF1 to P301S tau transgenic mice results in high and durable antibody expression, primarily in neurons. Hippocampal antibody levels achieved after AAV delivery were ∼50-fold more than those reported following repeated systemic administration. In contrast to systemic passive immunization, we observed markedly reduced (≥80-90%) hippocampal insoluble pathological tau species and neurofibrillary tangles following a single dose of AAV-vectored PHF1 compared with mice treated with an AAV-IgG control vector. Moreover, the hippocampal atrophy observed in untreated P301S mice was fully rescued by treatment with the AAV-vectored PHF1 antibody. Vectored passive immunotherapy with an anti-tau monoclonal antibody may represent a viable therapeutic strategy for treating or preventing such tauopathies as frontotemporal dementia, progressive supranuclear palsy, or Alzheimer's disease.
Significance statement: We have used an adeno-associated viral (AAV) vector to deliver the genes encoding an anti-phospho-tau monoclonal antibody, PHF1, directly to the brain of mice that develop neurodegeneration due to a tau mutation that causes frontotemporal dementia (FTD). When administered systemically, PHF1 has been shown to modestly reduce tau pathology and neurodegeneration. Since such antibodies do not readily cross the blood-brain barrier, we used an AAV vector to deliver antibody directly to the hippocampus and observed much higher antibody levels and a much greater reduction in tau pathology. Using AAV vectors to deliver antibodies like PHF1 directly to brain may constitute a novel approach to treating various neurodegenerative disorders, such as FTD and Alzheimer's disease.
Keywords: AAV vector; Alzheimer's disease; PHF-tau; anti-tau antibody; passive immunization; tauopathy.
Copyright © 2016 the authors 0270-6474/16/3612425-11$15.00/0.
Figures



References
-
- Ahmed Z, Cooper J, Murray TK, Garn K, McNaughton E, Clark H, Parhizkar S, Ward MA, Cavallini A, Jackson S, Bose S, Clavaguera F, Tolnay M, Lavenir I, Goedert M, Hutton ML, O'Neill MJ. A novel in vivo model of tau propagation with rapid and progressive neurofibrillary tangle pathology: the pattern of spread is determined by connectivity, not proximity. Acta Neuropathol. 2014;127:667–683. doi: 10.1007/s00401-014-1254-6. - DOI - PMC - PubMed
-
- Allen B, Ingram E, Takao M, Smith MJ, Jakes R, Virdee K, Yoshida H, Holzer M, Craxton M, Emson PC, Atzori C, Migheli A, Crowther RA, Ghetti B, Spillantini MG, Goedert M. Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein. J Neurosci. 2002;22:9340–9351. - PMC - PubMed
-
- Atwal JK, Chen Y, Chiu C, Mortensen DL, Meilandt WJ, Liu Y, Heise CE, Hoyte K, Luk W, Lu Y, Peng K, Wu P, Rouge L, Zhang Y, Lazarus RA, Scearce-Levie K, Wang W, Wu Y, Tessier-Lavigne M, Watts RJ. A therapeutic antibody targeting BACE1 inhibits amyloid-beta production in vivo. Sci Transl Med. 2011;3:84ra43. doi: 10.1126/scitranslmed.3002254. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
