Inhibition of Prolyl Oligopeptidase Restores Spontaneous Motor Behavior in the α-Synuclein Virus Vector-Based Parkinson's Disease Mouse Model by Decreasing α-Synuclein Oligomeric Species in Mouse Brain
- PMID: 27927963
- PMCID: PMC6601975
- DOI: 10.1523/JNEUROSCI.2309-16.2016
Inhibition of Prolyl Oligopeptidase Restores Spontaneous Motor Behavior in the α-Synuclein Virus Vector-Based Parkinson's Disease Mouse Model by Decreasing α-Synuclein Oligomeric Species in Mouse Brain
Abstract
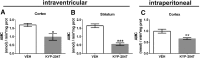
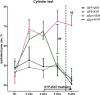
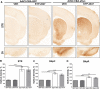
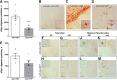
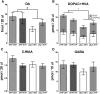
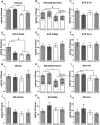
Decreased clearance of α-synuclein (aSyn) and aSyn protein misfolding and aggregation are seen as major factors in the pathogenesis of Parkinson's disease (PD) and other synucleinopathies that leads to disruption in neuronal function and eventually to cell death. Prolyl oligopeptidase (PREP) can accelerate the aSyn aggregation process, while inhibition of PREP by a small molecule inhibitor decreases aSyn oligomer formation and enhances its clearance via autophagy in different aSyn overexpressing cell types and in transgenic PD animal models. In this study, we investigated the impact of chronic PREP inhibition by a small molecule inhibitor, 4-phenylbutanoyl-l-prolyl-2(S)-cyanopyrrolidine (KYP-2047), on aSyn oligomerization, clearance, and underlying spontaneous motor behavior in a virus vector-based aSyn overexpression mouse model 4 weeks after aSyn microinjections and after the onset of symptomatic forepaw bias. Following 4 weeks of PREP inhibition, we saw an improved spontaneous forelimb use in mice that correlated with a decreased immunoreactivity against oligomer-specific forms of aSyn. Additionally, KYP-2047 had a trend to enhance dopaminergic systems activity. Our results suggest that PREP inhibition exhibits a beneficial effect on the aSyn clearance and aggregation in a virus mediated aSyn overexpression PD mouse model and that PREP inhibitors could be a novel therapeutic strategy for synucleinopathies.
Significance statement: Alpha-synuclein (aSyn) has been implicated in Parkinson's disease, with aSyn aggregates believed to exert toxic effects on neurons, while prolyl oligopeptidase (PREP) has been shown to interact with aSyn both in cells and cell free conditions, thus enhancing its aggregation. We demonstrate the possibility to abolish motor imbalance caused by aSyn viral vector injection with chronic 4 week PREP inhibition by a potent small-molecule PREP inhibitor, 4-phenylbutanoyl-l-prolyl-2(S)-cyanopyrrolidine (KYP-2047). Treatment was initiated postsymptomatically, 4 weeks after aSyn injection. KYP-2047-treated animals had a significantly decreased amount of oligomeric aSyn particles and improved dopamine system activity compared to control animals. To our knowledge, this is the first time viral overexpression of aSyn has been countered and movement impairments abolished after their onset.
Keywords: Parkinson's disease; prolyl oligopeptidase inhibitor; protein aggregation; serine protease; synuclein; synucleinopathies.
Copyright © 2016 the authors 0270-6474/16/3612485-13$15.00/0.
Figures

Similar articles
-
The beneficial effect of a prolyl oligopeptidase inhibitor, KYP-2047, on alpha-synuclein clearance and autophagy in A30P transgenic mouse.Neurobiol Dis. 2014 Aug;68:1-15. doi: 10.1016/j.nbd.2014.04.003. Epub 2014 Apr 16. Neurobiol Dis. 2014. PMID: 24746855 Free PMC article.
-
A prolyl oligopeptidase inhibitor, KYP-2047, reduces α-synuclein protein levels and aggregates in cellular and animal models of Parkinson's disease.Br J Pharmacol. 2012 Jun;166(3):1097-113. doi: 10.1111/j.1476-5381.2012.01846.x. Br J Pharmacol. 2012. PMID: 22233220 Free PMC article.
-
Prolyl oligopeptidase enhances α-synuclein dimerization via direct protein-protein interaction.J Biol Chem. 2015 Feb 20;290(8):5117-5126. doi: 10.1074/jbc.M114.592931. Epub 2015 Jan 2. J Biol Chem. 2015. PMID: 25555914 Free PMC article.
-
New tricks of prolyl oligopeptidase inhibitors - A common drug therapy for several neurodegenerative diseases.Biochem Pharmacol. 2019 Mar;161:113-120. doi: 10.1016/j.bcp.2019.01.013. Epub 2019 Jan 17. Biochem Pharmacol. 2019. PMID: 30660495 Review.
-
Prolyl oligopeptidase and dipeptidyl peptidase II/dipeptidyl peptidase IV ratio in the cerebrospinal fluid in Parkinson's disease: historical overview and future prospects.J Neural Transm (Vienna). 2017 Jun;124(6):739-744. doi: 10.1007/s00702-016-1604-8. Epub 2016 Aug 8. J Neural Transm (Vienna). 2017. PMID: 27503084 Review.
Cited by
-
Precision Medicine in Parkinson's Disease: From Genetic Risk Signals to Personalized Therapy.Brain Sci. 2022 Sep 28;12(10):1308. doi: 10.3390/brainsci12101308. Brain Sci. 2022. PMID: 36291241 Free PMC article. Review.
-
Therapeutic Effect of Prolyl Endopeptidase Inhibitor in High-fat Diet-induced Metabolic Dysfunction-associated Fatty Liver Disease.J Clin Transl Hepatol. 2023 Oct 28;11(5):1035-1049. doi: 10.14218/JCTH.2022.00110. Epub 2023 Apr 19. J Clin Transl Hepatol. 2023. PMID: 37577240 Free PMC article.
-
2-Imidazole as a Substitute for the Electrophilic Group Gives Highly Potent Prolyl Oligopeptidase Inhibitors.ACS Med Chem Lett. 2021 Sep 17;12(10):1578-1584. doi: 10.1021/acsmedchemlett.1c00399. eCollection 2021 Oct 14. ACS Med Chem Lett. 2021. PMID: 34671446 Free PMC article.
-
Prolyl Endopeptidase-Like Facilitates the α-Synuclein Aggregation Seeding, and This Effect Is Reverted by Serine Peptidase Inhibitor PMSF.Biomolecules. 2020 Jun 25;10(6):962. doi: 10.3390/biom10060962. Biomolecules. 2020. PMID: 32630529 Free PMC article.
-
Abnormal Expression of Prolyl Oligopeptidase (POP) and Its Catalytic Products Ac-SDKP Contributes to the Ovarian Fibrosis Change in Polycystic Ovary Syndrome (PCOS) Mice.Biomedicines. 2023 Jul 7;11(7):1927. doi: 10.3390/biomedicines11071927. Biomedicines. 2023. PMID: 37509566 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
