γδ T Cells Coexpressing Gut Homing α4β7 and αE Integrins Define a Novel Subset Promoting Intestinal Inflammation
- PMID: 27927968
- PMCID: PMC5225242
- DOI: 10.4049/jimmunol.1601060
γδ T Cells Coexpressing Gut Homing α4β7 and αE Integrins Define a Novel Subset Promoting Intestinal Inflammation
Abstract
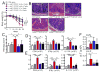
γδ T lymphocytes, dominant T cell subsets in the intestine, mediate both regulatory and pathogenic roles, yet the mechanisms underlying such opposing effects remain unclear. In this study, we identified a unique γδ T cell subset that coexpresses high levels of gut-homing integrins, CD103 and α4β7. They were exclusively found in the mesenteric lymph node after T cell-mediated colitis induction, and their appearance preceded the inflammation. Adoptive transfer of the CD103+α4β7high subsets enhanced Th1/Th17 T cell generation and accumulation in the intestine, and the disease severity. The level of generation correlated with the disease severity. Moreover, these cells were also found to be elevated in a spontaneous mouse model of ileitis. Based on the procolitogenic function, we referred to this subset as "inflammatory" γδ T cells. Targeting inflammatory γδ T cells may open a novel strategy to treat inflammatory diseases where γδ T cells play a pathogenic role including inflammatory bowel disease.
Copyright © 2017 by The American Association of Immunologists, Inc.
Figures







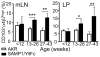
References
-
- Carding SR, Egan PJ. Gammadelta T cells: functional plasticity and heterogeneity. Nat Rev Immunol. 2002;2:336–345. - PubMed
-
- Chennupati V, Worbs T, Liu X, Malinarich FH, Schmitz S, Haas JD, Malissen B, Forster R, Prinz I. Intra- and intercompartmental movement of gammadelta T cells: intestinal intraepithelial and peripheral gammadelta T cells represent exclusive nonoverlapping populations with distinct migration characteristics. Journal of immunology. 2010;185:5160–5168. - PubMed
-
- Edelblum KL, Shen L, Weber CR, Marchiando AM, Clay BS, Wang Y, Prinz I, Malissen B, Sperling AI, Turner JR. Dynamic migration of gammadelta intraepithelial lymphocytes requires occludin. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:7097–7102. - PMC - PubMed
-
- Hayday A, Tigelaar R. Immunoregulation in the tissues by gammadelta T cells. Nat Rev Immunol. 2003;3:233–242. - PubMed
-
- Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, Girardi M, Borst J, Hayday AC, Pennington DJ, Silva-Santos B. CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nature immunology. 2009;10:427–436. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

