Cryo-electron Microscopy Study of the Genome Release of the Dicistrovirus Israeli Acute Bee Paralysis Virus
- PMID: 27928006
- PMCID: PMC5286892
- DOI: 10.1128/JVI.02060-16
Cryo-electron Microscopy Study of the Genome Release of the Dicistrovirus Israeli Acute Bee Paralysis Virus
Abstract
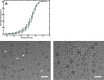
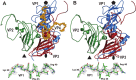
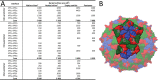
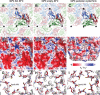
Viruses of the family Dicistroviridae can cause substantial economic damage by infecting agriculturally important insects. Israeli acute bee paralysis virus (IAPV) causes honeybee colony collapse disorder in the United States. High-resolution molecular details of the genome delivery mechanism of dicistroviruses are unknown. Here we present a cryo-electron microscopy analysis of IAPV virions induced to release their genomes in vitro We determined structures of full IAPV virions primed to release their genomes to a resolution of 3.3 Å and of empty capsids to a resolution of 3.9 Å. We show that IAPV does not form expanded A particles before genome release as in the case of related enteroviruses of the family Picornaviridae The structural changes observed in the empty IAPV particles include detachment of the VP4 minor capsid proteins from the inner face of the capsid and partial loss of the structure of the N-terminal arms of the VP2 capsid proteins. Unlike the case for many picornaviruses, the empty particles of IAPV are not expanded relative to the native virions and do not contain pores in their capsids that might serve as channels for genome release. Therefore, rearrangement of a unique region of the capsid is probably required for IAPV genome release.
Importance: Honeybee populations in Europe and North America are declining due to pressure from pathogens, including viruses. Israeli acute bee paralysis virus (IAPV), a member of the family Dicistroviridae, causes honeybee colony collapse disorder in the United States. The delivery of virus genomes into host cells is necessary for the initiation of infection. Here we present a structural cryo-electron microscopy analysis of IAPV particles induced to release their genomes. We show that genome release is not preceded by an expansion of IAPV virions as in the case of related picornaviruses that infect vertebrates. Furthermore, minor capsid proteins detach from the capsid upon genome release. The genome leaves behind empty particles that have compact protein shells.
Keywords: Aparavirus; Apis mellifera; CCD; Dicistroviridae; Picornavirales; capsid; colony collapse disorder; cryo; electron microscopy; empty; genome; honey bee; honeybee; release; structure; uncoating; virion; virus.
Copyright © 2017 Mullapudi et al.
Figures

Similar articles
-
Virion Structure of Israeli Acute Bee Paralysis Virus.J Virol. 2016 Aug 26;90(18):8150-9. doi: 10.1128/JVI.00854-16. Print 2016 Sep 15. J Virol. 2016. PMID: 27384649 Free PMC article.
-
Cryo-EM study of slow bee paralysis virus at low pH reveals iflavirus genome release mechanism.Proc Natl Acad Sci U S A. 2017 Jan 17;114(3):598-603. doi: 10.1073/pnas.1616562114. Epub 2017 Jan 4. Proc Natl Acad Sci U S A. 2017. PMID: 28053231 Free PMC article.
-
Virion Structure of Black Queen Cell Virus, a Common Honeybee Pathogen.J Virol. 2017 Feb 28;91(6):e02100-16. doi: 10.1128/JVI.02100-16. Print 2017 Mar 15. J Virol. 2017. PMID: 28077635 Free PMC article.
-
The Acute bee paralysis virus-Kashmir bee virus-Israeli acute paralysis virus complex.J Invertebr Pathol. 2010 Jan;103 Suppl 1:S30-47. doi: 10.1016/j.jip.2009.06.014. Epub 2009 Nov 11. J Invertebr Pathol. 2010. PMID: 19909972 Review.
-
Virion structures and genome delivery of honeybee viruses.Curr Opin Virol. 2020 Dec;45:17-24. doi: 10.1016/j.coviro.2020.06.007. Epub 2020 Jul 14. Curr Opin Virol. 2020. PMID: 32679289 Review.
Cited by
-
Capsid opening enables genome release of iflaviruses.Sci Adv. 2021 Jan 1;7(1):eabd7130. doi: 10.1126/sciadv.abd7130. Print 2021 Jan. Sci Adv. 2021. PMID: 33523856 Free PMC article.
-
Multiscale modelization in a small virus: Mechanism of proton channeling and its role in triggering capsid disassembly.PLoS Comput Biol. 2018 Apr 16;14(4):e1006082. doi: 10.1371/journal.pcbi.1006082. eCollection 2018 Apr. PLoS Comput Biol. 2018. PMID: 29659564 Free PMC article.
-
Virion structure and in vitro genome release mechanism of dicistrovirus Kashmir bee virus.J Virol. 2021 May 10;95(11):e01950-20. doi: 10.1128/JVI.01950-20. Epub 2021 Mar 3. J Virol. 2021. PMID: 33658338 Free PMC article.
-
The Israeli acute paralysis virus IRES captures host ribosomes by mimicking a ribosomal state with hybrid tRNAs.EMBO J. 2019 Oct 4;38(21):e102226. doi: 10.15252/embj.2019102226. Epub 2019 Oct 14. EMBO J. 2019. PMID: 31609474 Free PMC article.
-
Conformational Changes and Nuclear Entry of Porcine Circovirus without Disassembly.J Virol. 2019 Sep 30;93(20):e00824-19. doi: 10.1128/JVI.00824-19. Print 2019 Oct 15. J Virol. 2019. PMID: 31341057 Free PMC article.
References
-
- Biesmeijer JC, Roberts SP, Reemer M, Ohlemuller R, Edwards M, Peeters T, Schaffers AP, Potts SG, Kleukers R, Thomas CD, Settele J, Kunin WE. 2006. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 313:351–354. doi:10.1126/science.1127863. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

