Herpes Simplex Virus 1 UL41 Protein Suppresses the IRE1/XBP1 Signal Pathway of the Unfolded Protein Response via Its RNase Activity
- PMID: 27928013
- PMCID: PMC5286897
- DOI: 10.1128/JVI.02056-16
Herpes Simplex Virus 1 UL41 Protein Suppresses the IRE1/XBP1 Signal Pathway of the Unfolded Protein Response via Its RNase Activity
Abstract
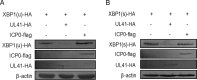
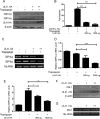
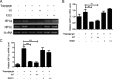
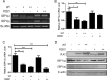
During viral infection, accumulation of viral proteins can cause stress in the endoplasmic reticulum (ER) and trigger the unfolded protein response (UPR) to restore ER homeostasis. The inositol-requiring enzyme 1 (IRE1)-dependent pathway is the most conserved of the three UPR signal pathways. Upon activation, IRE1 splices out an intron from the unspliced inactive form of X box binding protein 1 [XBP1(u)] mRNA and produces a transcriptionally potent spliced form [XBP1(s)]. Previous studies have reported that the IRE1/XBP1 pathway is inhibited upon herpes simplex virus 1 (HSV-1) infection; however, the underlying molecular mechanism is still elusive. Here, we uncovered a role of the HSV-1 UL41 protein in inhibiting the IRE1/XBP1 signal pathway. Ectopic expression of UL41 decreased the expression of XBP1 and blocked XBP1 splicing activation induced by the ER stress inducer thapsigargin. Wild-type (WT) HSV-1, but not the UL41-null mutant HSV-1 (R2621), decreased XBP1 mRNA induced by thapsigargin. Nevertheless, infection with both WT HSV-1 and R2621 without drug pretreatment could reduce the mRNA and protein levels of XBP1(s), and additional mechanisms might contribute to this inhibition of XBP1(s) during R2621 infection. Taking these findings together, our results reveal XBP1 as a novel target of UL41 and provide insights into the mechanism by which HSV-1 modulates the IRE1/XBP1 pathway.
Importance: During viral infection, viruses hijack the host translation apparatus to produce large amounts of viral proteins, which leads to ER stress. To restore ER homeostasis, cells initiate the UPR to alleviate the effects of ER stress. The IRE1/XBP1 pathway is the most conserved UPR branch, and it activates ER-associated protein degradation (ERAD) to reduce the ER load. The IRE1/XBP1 branch is repressed during HSV-1 infection, but little is known about the underlying molecular mechanism. Our results show for the first time that UL41 suppresses the IRE1/XBP1 signal pathway by reducing the accumulation of XBP1 mRNA, and characterization of the underlying molecular mechanism provides new insight into the modulation of UPR by HSV-1.
Keywords: HSV-1; UL41; UPR; XBP1.
Copyright © 2017 American Society for Microbiology.
Figures
Similar articles
-
Opposite Roles of RNase and Kinase Activities of Inositol-Requiring Enzyme 1 (IRE1) on HSV-1 Replication.Viruses. 2017 Aug 23;9(9):235. doi: 10.3390/v9090235. Viruses. 2017. PMID: 28832521 Free PMC article.
-
Unfolded protein response transducer IRE1-mediated signaling independent of XBP1 mRNA splicing is not required for growth and development of medaka fish.Elife. 2017 Sep 27;6:e26845. doi: 10.7554/eLife.26845. Elife. 2017. PMID: 28952924 Free PMC article.
-
Marburg virus regulates the IRE1/XBP1-dependent unfolded protein response to ensure efficient viral replication.Emerg Microbes Infect. 2019;8(1):1300-1313. doi: 10.1080/22221751.2019.1659552. Emerg Microbes Infect. 2019. PMID: 31495285 Free PMC article.
-
The molecular mechanism and functional diversity of UPR signaling sensor IRE1.Life Sci. 2021 Jan 15;265:118740. doi: 10.1016/j.lfs.2020.118740. Epub 2020 Nov 11. Life Sci. 2021. PMID: 33188833 Review.
-
X-box binding protein 1 (XBP1) function in diseases.Cell Biol Int. 2021 Apr;45(4):731-739. doi: 10.1002/cbin.11533. Epub 2020 Dec 25. Cell Biol Int. 2021. PMID: 33325615 Review.
Cited by
-
Viral Infections and Their Ability to Modulate Endoplasmic Reticulum Stress Response Pathways.Viruses. 2024 Sep 30;16(10):1555. doi: 10.3390/v16101555. Viruses. 2024. PMID: 39459886 Free PMC article. Review.
-
Pseudorabies virus VHS protein abrogates interferon responses by blocking NF-κB and IRF3 nuclear translocation.Virol Sin. 2024 Aug;39(4):587-599. doi: 10.1016/j.virs.2024.05.009. Epub 2024 May 30. Virol Sin. 2024. PMID: 38823782 Free PMC article.
-
Influenza A viruses balance ER stress with host protein synthesis shutoff.Proc Natl Acad Sci U S A. 2021 Sep 7;118(36):e2024681118. doi: 10.1073/pnas.2024681118. Proc Natl Acad Sci U S A. 2021. PMID: 34479996 Free PMC article.
-
Nuclear-cytoplasmic compartmentalization of the herpes simplex virus 1 infected cell transcriptome is co-ordinated by the viral endoribonuclease vhs and cofactors to facilitate the translation of late proteins.PLoS Pathog. 2018 Nov 26;14(11):e1007331. doi: 10.1371/journal.ppat.1007331. eCollection 2018 Nov. PLoS Pathog. 2018. PMID: 30475899 Free PMC article.
-
Pathological consequences of the unfolded protein response and downstream protein disulphide isomerases in pulmonary viral infection and disease.J Biochem. 2020 Feb 1;167(2):173-184. doi: 10.1093/jb/mvz101. J Biochem. 2020. PMID: 31790139 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

