The E3 ubiquitin ligase TRIM31 attenuates NLRP3 inflammasome activation by promoting proteasomal degradation of NLRP3
- PMID: 27929086
- PMCID: PMC5155141
- DOI: 10.1038/ncomms13727
The E3 ubiquitin ligase TRIM31 attenuates NLRP3 inflammasome activation by promoting proteasomal degradation of NLRP3
Abstract
The NLRP3 inflammasome has a fundamental role in host defence against microbial pathogens and its deregulation may cause diverse inflammatory diseases. NLRP3 protein expression is a rate-limiting step for inflammasome activation, thus its expression must be tightly controlled to maintain immune homeostasis and avoid detrimental effects. However, how NLRP3 expression is regulated remains largely unknown. In this study, we identify E3 ubiquitin ligase TRIM31 as a feedback suppressor of NLRP3 inflammasome. TRIM31 directly binds to NLRP3, promotes K48-linked polyubiquitination and proteasomal degradation of NLRP3. Consequently, TRIM31 deficiency enhances NLRP3 inflammasome activation and aggravates alum-induced peritonitis in vivo. Furthermore, TRIM31 deficiency attenuates the severity of dextran sodium sulfate (DSS)-induced colitis, an inflammatory bowel diseases model in which NLRP3 possesses protective roles. Thus, our research describes a mechanism by which TRIM31 limits NLRP3 inflammasome activity under physiological conditions and suggests TRIM31 as a potential therapeutic target for the intervention of NLRP3 inflammasome related diseases.
Figures




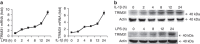



References
-
- Schroder K. & Tschopp J. The inflammasomes. Cell 140, 821–832 (2010). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

