Glymphatic solute transport does not require bulk flow
- PMID: 27929105
- PMCID: PMC5144134
- DOI: 10.1038/srep38635
Glymphatic solute transport does not require bulk flow
Abstract
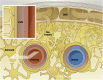
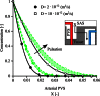
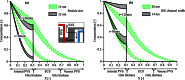
Observations of fast transport of fluorescent tracers in mouse brains have led to the hypothesis of bulk water flow directed from arterial to venous paravascular spaces (PVS) through the cortical interstitium. At the same time, there is evidence for interstitial solute transport by diffusion rather than by directed bulk fluid motion. It has been shown that the two views may be consolidated by intracellular water flow through astrocyte networks combined with mainly diffusive extracellular transport of solutes. This requires the presence of a driving force that has not been determined to date, but for which arterial pulsation has been suggested as the origin. Here we show that arterial pulsation caused by pulse wave propagation is an unlikely origin of this hypothetical driving force. However, we further show that such pulsation may still lead to fast para-arterial solute transport through dispersion, that is, through the combined effect of local mixing and diffusion in the para-arterial space.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Cserr H. & Patlak C. Secretion and bulk flow of interstitial fluid. In: Physiology and pharmacology of the blood-brain barrier, 245–261, Springer (1992).
-
- Kurtcuoglu V., Soellinger M., Summers P., Poulikakos D. & Boesiger P. Mixing and modes of mass transfer in the third cerebral ventricle: a computational analysis. J Biomech 129, 695–702 (2007). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

