Dual targeting of glutaminase 1 and thymidylate synthase elicits death synergistically in NSCLC
- PMID: 27929535
- PMCID: PMC5261012
- DOI: 10.1038/cddis.2016.404
Dual targeting of glutaminase 1 and thymidylate synthase elicits death synergistically in NSCLC
Abstract
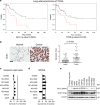
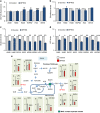
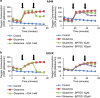
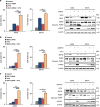
Glutaminase 1 (GLS1) expression is increased in non-small cell lung cancer (NSCLC). GLS1 knockdown using siRNA or inhibition using bis-2-(5-phenylacetamido-1,3,4-thiadiazol-2-yl)ethyl sulfide (BPTES) induced cell cycle arrest with significant reduction of ATP level while levels of reactive oxygen species or glutathione were not affected in NSCLC cell lines. Recently we found that NSCLC significantly depends on cytosol NADH for ATP production. GLS1 remarkably contributes to ATP production through transferring cytosolic NADH into mitochondria via malate-aspartate shuttle by supply of glutamate in NSCLC. Regulation of malate-aspartate shuttle by knockdown or inhibition of glutamic-oxaloacetic transaminase 2 or malate dehydrogenase 2 mimicked GLS1 knockdown, which induced cell death with ATP reduction in NSCLC. Therefore, GLS1 inhibition induced cell cycle arrest with ATP depletion by glutamate reduction. Dual inhibition with BPTES and thymidylate synthase inhibitor, 5-fluorouracil (5-FU), elicits cell death synergistically through cell cycle arrest in NSCLC. A preclinical xenograft model of NSCLC showed remarkable anti-tumour effect synergistically in the BPTES and 5-FU dual therapy group.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

