FGF23 Is Not Required to Regulate Fetal Phosphorus Metabolism but Exerts Effects Within 12 Hours After Birth
- PMID: 27929669
- PMCID: PMC5413075
- DOI: 10.1210/en.2016-1369
FGF23 Is Not Required to Regulate Fetal Phosphorus Metabolism but Exerts Effects Within 12 Hours After Birth
Abstract
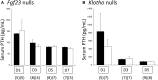
Loss of fibroblast growth factor-23 (FGF23) causes hyperphosphatemia, extraskeletal calcifications, and early mortality; excess FGF23 causes hypophosphatemia with rickets or osteomalacia. However, FGF23 may not be important during fetal development. FGF23 deficiency (Fgf23 null) and FGF23 excess (Phex null) did not alter fetal phosphorus or skeletal parameters. In this study, we further tested our hypothesis that FGF23 is not essential for fetal phosphorus regulation but becomes important after birth. Although coreceptor Klotho null adults have extremely high FGF23 concentrations, intact FGF23 was normal in Klotho null fetuses, as were fetal phosphorus and skeletal parameters and placental and renal expression of FGF23 target genes. Pth/Fgf23 double mutants had the same elevation in serum phosphorus as Pth null fetuses, as compared with normal serum phosphorus in Fgf23 nulls. We examined the postnatal time courses of Fgf23 null, Klotho null, and Phex null mice. Fgf23 nulls and Klotho nulls were normal at birth, but developed hyperphosphatemia, increased renal expression of NaPi2a and NaPi2c, and reduced renal phosphorus excretion between 5 and 7 days after birth. Parathyroid hormone remained normal. In contrast, excess FGF23 exerted effects in Phex null males within 12 hours after birth, with the development of hypophosphatemia, reduced renal expression of NaPi2a and NaPi2c, and increased renal phosphorus excretion. In conclusion, although FGF23 is present in the fetal circulation at levels that may equal adult values, and there is robust expression of FGF23 target genes in placenta and fetal kidneys, FGF23 itself is not an important regulator of fetal phosphorous metabolism.
Copyright © 2017 by the Endocrine Society.
Figures




Similar articles
-
Neither absence nor excess of FGF23 disturbs murine fetal-placental phosphorus homeostasis or prenatal skeletal development and mineralization.Endocrinology. 2014 May;155(5):1596-605. doi: 10.1210/en.2013-2061. Epub 2014 Mar 6. Endocrinology. 2014. PMID: 24601885 Free PMC article.
-
Murine Fetal Serum Phosphorus is Set Independent of FGF23 and PTH, Except in the Presence of Maternal Phosphate Loading.Endocrinology. 2021 Jan 1;162(1):bqaa202. doi: 10.1210/endocr/bqaa202. Endocrinology. 2021. PMID: 33150413 Free PMC article.
-
In vivo evidence for an interplay of FGF23/Klotho/PTH axis on the phosphate handling in renal proximal tubules.Am J Physiol Renal Physiol. 2018 Nov 1;315(5):F1261-F1270. doi: 10.1152/ajprenal.00650.2017. Epub 2018 Jul 11. Am J Physiol Renal Physiol. 2018. PMID: 29993278 Free PMC article.
-
Vitamin D and type II sodium-dependent phosphate cotransporters.Contrib Nephrol. 2013;180:86-97. doi: 10.1159/000346786. Epub 2013 May 6. Contrib Nephrol. 2013. PMID: 23652552 Review.
-
PHEX, FGF23, DMP1 and beyond.Curr Opin Nephrol Hypertens. 2008 Jul;17(4):357-62. doi: 10.1097/MNH.0b013e3282fd6e5b. Curr Opin Nephrol Hypertens. 2008. PMID: 18660670 Review.
Cited by
-
Vibrational spectroscopic analysis of hydroxyapatite in HYP mice and individuals with X-linked hypophosphatemia.Ther Adv Chronic Dis. 2018 Oct 11;9(12):268-281. doi: 10.1177/2040622318804753. eCollection 2018. Ther Adv Chronic Dis. 2018. PMID: 30719271 Free PMC article.
-
Prenatal hyperechogenic kidneys in three cases of infantile hypercalcemia associated with SLC34A1 mutations.Pediatr Nephrol. 2018 Oct;33(10):1723-1729. doi: 10.1007/s00467-018-3998-z. Epub 2018 Jun 29. Pediatr Nephrol. 2018. PMID: 29959532
-
Maternal excess dietary phosphate intake in the periconceptional period is a potential risk for mineral disorders in offspring mice.Sci Rep. 2025 Mar 14;15(1):8844. doi: 10.1038/s41598-025-91717-2. Sci Rep. 2025. PMID: 40087383 Free PMC article.
-
Hormonal regulation of biomineralization.Nat Rev Endocrinol. 2021 May;17(5):261-275. doi: 10.1038/s41574-021-00477-2. Epub 2021 Mar 16. Nat Rev Endocrinol. 2021. PMID: 33727709 Review.
-
Mineral Metabolism in Children: Interrelation between Vitamin D and FGF23.Int J Mol Sci. 2023 Apr 3;24(7):6661. doi: 10.3390/ijms24076661. Int J Mol Sci. 2023. PMID: 37047636 Free PMC article. Review.
References
-
- Silver J, Naveh-Many T. FGF23 and the parathyroid glands. Pediatr Nephrol. 2010;25(11):2241–2245. - PubMed
-
- Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19(3):429–435. - PubMed
-
- Wild R, Gerasimaite R, Jung JY, Truffault V, Pavlovic I, Schmidt A, Saiardi A, Jessen HJ, Poirier Y, Hothorn M, Mayer A. Control of eukaryotic phosphate homeostasis by inositol polyphosphate sensor domains. Science. 2016;352(6288):986–990. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

