Gamma motor neurons survive and exacerbate alpha motor neuron degeneration in ALS
- PMID: 27930290
- PMCID: PMC5187676
- DOI: 10.1073/pnas.1605210113
Gamma motor neurons survive and exacerbate alpha motor neuron degeneration in ALS
Abstract
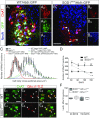
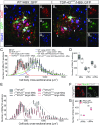
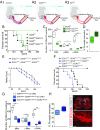
The molecular and cellular basis of selective motor neuron (MN) vulnerability in amyotrophic lateral sclerosis (ALS) is not known. In genetically distinct mouse models of familial ALS expressing mutant superoxide dismutase-1 (SOD1), TAR DNA-binding protein 43 (TDP-43), and fused in sarcoma (FUS), we demonstrate selective degeneration of alpha MNs (α-MNs) and complete sparing of gamma MNs (γ-MNs), which selectively innervate muscle spindles. Resistant γ-MNs are distinct from vulnerable α-MNs in that they lack synaptic contacts from primary afferent (IA) fibers. Elimination of these synapses protects α-MNs in the SOD1 mutant, implicating this excitatory input in MN degeneration. Moreover, reduced IA activation by targeted reduction of γ-MNs in SOD1G93A mutants delays symptom onset and prolongs lifespan, demonstrating a pathogenic role of surviving γ-MNs in ALS. This study establishes the resistance of γ-MNs as a general feature of ALS mouse models and demonstrates that synaptic excitation of MNs within a complex circuit is an important determinant of relative vulnerability in ALS.
Keywords: ALS; fusimotor; gamma motor neuron; motor neuron disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Robberecht W, Philips T. The changing scene of amyotrophic lateral sclerosis. Nat Rev Neurosci. 2013;14(4):248–264. - PubMed
-
- Carvalho M, Schwartz MS, Swash M. Involvement of the external anal sphincter in amyotrophic lateral sclerosis. Muscle Nerve. 1995;18(8):848–853. - PubMed
-
- Ferrucci M, et al. A systematic study of brainstem motor nuclei in a mouse model of ALS, the effects of lithium. Neurobiol Dis. 2010;37(2):370–383. - PubMed
-
- Iwata M, Hirano A. Sparing of the Onufrowicz nucleus in sacral anterior horn lesions. Ann Neurol. 1978;4(3):245–249. - PubMed
-
- Kanning KC, Kaplan A, Henderson CE. Motor neuron diversity in development and disease. Annu Rev Neurosci. 2010;33:409–440. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

