UV-B photoreceptor-mediated protection of the photosynthetic machinery in Chlamydomonas reinhardtii
- PMID: 27930292
- PMCID: PMC5187749
- DOI: 10.1073/pnas.1607695114
UV-B photoreceptor-mediated protection of the photosynthetic machinery in Chlamydomonas reinhardtii
Abstract
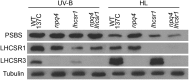
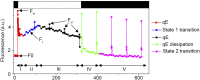
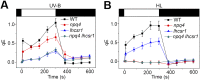
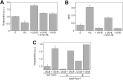
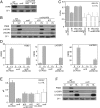
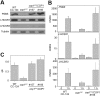
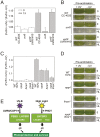
Life on earth is dependent on the photosynthetic conversion of light energy into chemical energy. However, absorption of excess sunlight can damage the photosynthetic machinery and limit photosynthetic activity, thereby affecting growth and productivity. Photosynthetic light harvesting can be down-regulated by nonphotochemical quenching (NPQ). A major component of NPQ is qE (energy-dependent nonphotochemical quenching), which allows dissipation of light energy as heat. Photodamage peaks in the UV-B part of the spectrum, but whether and how UV-B induces qE are unknown. Plants are responsive to UV-B via the UVR8 photoreceptor. Here, we report in the green alga Chlamydomonas reinhardtii that UVR8 induces accumulation of specific members of the light-harvesting complex (LHC) superfamily that contribute to qE, in particular LHC Stress-Related 1 (LHCSR1) and Photosystem II Subunit S (PSBS). The capacity for qE is strongly induced by UV-B, although the patterns of qE-related proteins accumulating in response to UV-B or to high light are clearly different. The competence for qE induced by acclimation to UV-B markedly contributes to photoprotection upon subsequent exposure to high light. Our study reveals an anterograde link between photoreceptor-mediated signaling in the nucleocytosolic compartment and the photoprotective regulation of photosynthetic activity in the chloroplast.
Keywords: LHCSR1; PSBS; UV-B photoreceptor; nonphotochemical quenching; photoprotection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Li Z, Wakao S, Fischer BB, Niyogi KK. Sensing and responding to excess light. Annu Rev Plant Biol. 2009;60:239–260. - PubMed
-
- Erickson E, Wakao S, Niyogi KK. Light stress and photoprotection in Chlamydomonas reinhardtii. Plant J. 2015;82(3):449–465. - PubMed
-
- Minagawa J, Tokutsu R. Dynamic regulation of photosynthesis in Chlamydomonas reinhardtii. Plant J. 2015;82(3):413–428. - PubMed
-
- Niyogi KK, Truong TB. Evolution of flexible non-photochemical quenching mechanisms that regulate light harvesting in oxygenic photosynthesis. Curr Opin Plant Biol. 2013;16(3):307–314. - PubMed
-
- Wobbe L, Bassi R, Kruse O. Multi-level light capture control in plants and green algae. Trends Plant Sci. 2016;21(1):55–68. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

